Col4a2 Mutations Contribute to Infantile Epileptic Spasm Syndrome and Neuroinflammation
- PMID: 39006838
- PMCID: PMC11241092
- DOI: 10.7150/ijms.97164
Col4a2 Mutations Contribute to Infantile Epileptic Spasm Syndrome and Neuroinflammation
Abstract
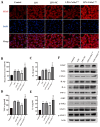
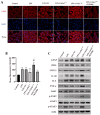
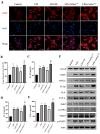
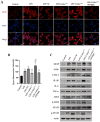
There are more than 70 million people worldwide living with epilepsy, with most experiencing the onset of epilepsy in childhood. Despite the availability of more than 20 anti-seizure medications, approximately 30% of epilepsy patients continue to experience unsatisfactory treatment outcomes. This situation places a heavy burden on patients' families and society. Childhood epilepsy is a significant chronic neurological disease that is closely related to genetics. Col4a2, the gene encoding the α2 chain of type IV collagen, is known to be associated with multiple diseases due to missense mutations. The Col4a2 variant of collagen type IV is associated with various phenotypes, including prenatal and neonatal intracranial hemorrhage, porencephaly, porencephaly with cataracts, focal cortical dysplasia, schizencephaly, strokes in childhood and adolescence, and sporadic delayed hemorrhagic stroke. Although epilepsy is recognized as a clinical manifestation of porencephaly, the specific mechanism of Col4a2-related epileptic phenotypes remains unclear. A total of 8 patients aged 2 years and 2 months to 18 years who were diagnosed with Col4a2-related infantile epileptic spasm syndrome were analyzed. The seizure onset age ranged from 3 to 10 months. Initial EEG results revealed hypsarrhythmia or multiple and multifocal sharp waves, spike waves, sharp slow waves, or spike slow waves. Elevated levels of the cytokines IL-1β (32.23±12.58 pg/ml) and IL-6 (45.12±16.03 pg/ml) were detected in the cerebrospinal fluid of these patients without any signs of infection. Following antiseizure treatment, decreased IL-1β and IL-6 levels in the cerebrospinal fluid were noted when seizures were under control. Furthermore, we aimed to investigate the role of Col4a2 mutations in the development of epilepsy. Through the use of immunofluorescence assays, ELISA, and Western blotting, we examined astrocyte activity and the expression of inflammatory cytokines such as IL-1β, IL-6, and TNF-α after overexpressing an unreported Col4a2 (c.1838G>T) mutant in CTX-TNA cells and primary astrocytes. We found that the levels of the inflammatory factors IL-1β, IL-6, and TNF-α were increased in both CTX-TNA cells (ELISA: p = 0.0087, p<0.001, p<0.001, respectively) and primary astrocytes (ELISA: p = 0.0275, p<0.001, p<0.001, respectively). Additionally, we conducted a preliminary investigation of the role of the JAK/STAT pathway in Col4a2 mutation-associated epilepsy. Col4a2 mutation stimulated astrocyte activation, increasing iNOS, COX-2, IL-1β, IL-6, and TNF-α levels in both CTX-TNA cells and primary astrocytes. This mutation also activated the JAK/STAT signaling pathway, leading to increased phosphorylation of JAK2 and STAT3. Treatment with the JAK/STAT inhibitor WP1066 effectively counteracted this effect in primary astrocytes and CTX-TNA cells. To date, the genes who mutations are known to cause developmental and epileptic encephalopathies (DEEs) are predominantly grouped into six subtypes according to function. Our study revealed that an unreported mutation site Col4a2Mut (c.1838G>T) of which can cause neuroinflammation, may be a type VII DEE-causing gene.
Keywords: Col4a2; astrocytes; infantile epileptic spasm syndrome; neuroinflammation.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
Similar articles
-
[Genotype and phenotype of WWOX gene related developmental and epileptic encephalopathy].Zhonghua Er Ke Za Zhi. 2024 Aug 2;62(8):752-757. doi: 10.3760/cma.j.cn112140-20240229-00135. Zhonghua Er Ke Za Zhi. 2024. PMID: 39039877 Chinese.
-
COL4A2 mutation associated with familial porencephaly and small-vessel disease.Eur J Hum Genet. 2012 Aug;20(8):844-51. doi: 10.1038/ejhg.2012.20. Epub 2012 Feb 15. Eur J Hum Genet. 2012. PMID: 22333902 Free PMC article.
-
[Clinical and genetic characteristics of children with STXBP1 encephalopathy].Zhonghua Er Ke Za Zhi. 2020 Jun 2;58(6):493-498. doi: 10.3760/cma.j.cn112140-20191028-00683. Zhonghua Er Ke Za Zhi. 2020. PMID: 32521962 Chinese.
-
Pharmacological modulation of cytokines correlating neuroinflammatory cascades in epileptogenesis.Mol Biol Rep. 2022 Feb;49(2):1437-1452. doi: 10.1007/s11033-021-06896-8. Epub 2021 Nov 9. Mol Biol Rep. 2022. PMID: 34751915 Review.
-
[Early infantile epileptic encephalopathy caused by PACS2 gene variation: three cases report and literature review].Zhonghua Er Ke Za Zhi. 2021 Jul 2;59(7):594-599. doi: 10.3760/cma.j.cn112140-20201122-01047. Zhonghua Er Ke Za Zhi. 2021. PMID: 34405643 Review. Chinese.
References
-
- Thijs RD, Surges R, O'Brien TJ, Sander JW. Epilepsy in adults. The Lancet. 2019;393:689–701. - PubMed
-
- Pitkänen A, Lukasiuk K. Mechanisms of epileptogenesis and potential treatment targets. The Lancet Neurology. 2011;10:173–86. - PubMed
-
- Terrone G, Balosso S, Pauletti A, Ravizza T, Vezzani A. Inflammation and reactive oxygen species as disease modifiers in epilepsy. Neuropharmacology. 2020;167:107742. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

