This is a preprint.
Multiple mechanisms of aminoglycoside ototoxicity are distinguished by subcellular localization of action
- PMID: 39005374
- PMCID: PMC11244871
- DOI: 10.1101/2024.05.30.596537
Multiple mechanisms of aminoglycoside ototoxicity are distinguished by subcellular localization of action
Update in
-
Multiple mechanisms of aminoglycoside ototoxicity are distinguished by subcellular localization of action.Front Neurol. 2024 Nov 14;15:1480435. doi: 10.3389/fneur.2024.1480435. eCollection 2024. Front Neurol. 2024. PMID: 39610699 Free PMC article.
Abstract
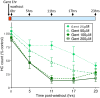
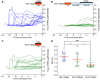
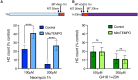
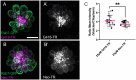
Mechanosensory hair cells of the inner ears and lateral line of vertebrates display heightened vulnerability to environmental insult, with damage resulting in hearing and balance disorders. An important example is hair cell loss due to exposure to toxic agents including therapeutic drugs such as the aminoglycoside antibiotics such as neomycin and gentamicin and antineoplastic agents. We describe two distinct cellular pathways for aminoglycoside-induced hair cell death in zebrafish lateral line hair cells. Neomycin exposure results in death from acute exposure with most cells dying within 1 hour of exposure. By contrast, exposure to gentamicin results primarily in delayed hair cell death, taking up to 24 hours for maximal effect. Washout experiments demonstrate that delayed death does not require continuous exposure, demonstrating two mechanisms where downstream responses differ in their timing. Acute damage is associated with mitochondrial calcium fluxes and can be alleviated by the mitochondrially-targeted antioxidant mitoTEMPO, while delayed death is independent of these factors. Conversely delayed death is associated with lysosomal accumulation and is reduced by altering endolysosomal function, while acute death is not sensitive to lysosomal manipulations. These experiments reveal the complexity of responses of hair cells to closely related compounds, suggesting that intervention focusing on early events rather than specific death pathways may be a successful therapeutic strategy.
Figures


Similar articles
-
Multiple mechanisms of aminoglycoside ototoxicity are distinguished by subcellular localization of action.Front Neurol. 2024 Nov 14;15:1480435. doi: 10.3389/fneur.2024.1480435. eCollection 2024. Front Neurol. 2024. PMID: 39610699 Free PMC article.
-
Genedrive kit for detecting single nucleotide polymorphism m.1555A>G in neonates and their mothers: a systematic review and cost-effectiveness analysis.Health Technol Assess. 2024 Oct;28(75):1-75. doi: 10.3310/TGAC4201. Health Technol Assess. 2024. PMID: 39487741 Free PMC article.
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Qualitative evidence synthesis informing our understanding of people's perceptions and experiences of targeted digital communication.Cochrane Database Syst Rev. 2019 Oct 23;10(10):ED000141. doi: 10.1002/14651858.ED000141. Cochrane Database Syst Rev. 2019. PMID: 31643081 Free PMC article.
-
Pharmacological treatments in panic disorder in adults: a network meta-analysis.Cochrane Database Syst Rev. 2023 Nov 28;11(11):CD012729. doi: 10.1002/14651858.CD012729.pub3. Cochrane Database Syst Rev. 2023. PMID: 38014714 Free PMC article. Review.
References
-
- Au S, Weiner ND, Schacht J. (1987) Aminoglycoside antibiotics preferentially increase permeability in phosphoinositide-containing membranes: a study with carboxyfluorescein in liposomes. Biochim Biophys Acta. 902:80–6. - PubMed
-
- Ballabio A, Bonifacino JS. (2020) Lysosomes as dynamic regulators of cell and organismal homeostasis. Nat Rev Mol Cell Biol. 21:101–118. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
