A New Application for Cenicriviroc, a Dual CCR2/CCR5 Antagonist, in the Treatment of Painful Diabetic Neuropathy in a Mouse Model
- PMID: 39000516
- PMCID: PMC11242565
- DOI: 10.3390/ijms25137410
A New Application for Cenicriviroc, a Dual CCR2/CCR5 Antagonist, in the Treatment of Painful Diabetic Neuropathy in a Mouse Model
Abstract
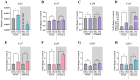
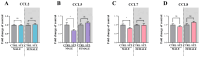
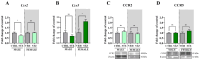
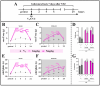
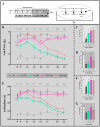
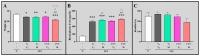
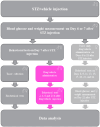
The ligands of chemokine receptors 2 and 5 (CCR2 and CCR5, respectively) are associated with the pathomechanism of neuropathic pain development, but their role in painful diabetic neuropathy remains unclear. Therefore, the aim of our study was to examine the function of these factors in the hypersensitivity accompanying diabetes. Additionally, we analyzed the analgesic effect of cenicriviroc (CVC), a dual CCR2/CCR5 antagonist, and its influence on the effectiveness of morphine. An increasing number of experimental studies have shown that targeting more than one molecular target is advantageous compared with the coadministration of individual pharmacophores in terms of their analgesic effect. The advantage of using bifunctional compounds is that they gain simultaneous access to two receptors at the same dose, positively affecting their pharmacokinetics and pharmacodynamics and consequently leading to improved analgesia. Experiments were performed on male and female Swiss albino mice with a streptozotocin (STZ, 200 mg/kg, i.p.) model of diabetic neuropathy. We found that the blood glucose level increased, and the mechanical and thermal hypersensitivity developed on the 7th day after STZ administration. In male mice, we observed increased mRNA levels of Ccl2, Ccl5, and Ccl7, while in female mice, we observed additional increases in Ccl8 and Ccl12 levels. We have demonstrated for the first time that a single administration of cenicriviroc relieves pain to a similar extent in male and female mice. Moreover, repeated coadministration of cenicriviroc with morphine delays the development of opioid tolerance, while the best and longest-lasting analgesic effect is achieved by repeated administration of cenicriviroc alone, which reduces pain hypersensitivity in STZ-exposed mice, and unlike morphine, no tolerance to the analgesic effects of CVC is observed until Day 15 of treatment. Based on these results, we suggest that targeting CCR2 and CCR5 with CVC is a potent therapeutic option for novel pain treatments in diabetic neuropathy patients.
Keywords: cenicriviroc; chemokines; diabetes; female; male; neuropathic pain.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflicts of interest.
Figures
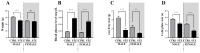
Similar articles
-
A Missing Puzzle in Preclinical Studies-Are CCR2, CCR5, and Their Ligands' Roles Similar in Obesity-Induced Hypersensitivity and Diabetic Neuropathy?-Evidence from Rodent Models and Clinical Studies.Int J Mol Sci. 2024 Oct 21;25(20):11323. doi: 10.3390/ijms252011323. Int J Mol Sci. 2024. PMID: 39457105 Free PMC article. Review.
-
Bidirectional Action of Cenicriviroc, a CCR2/CCR5 Antagonist, Results in Alleviation of Pain-Related Behaviors and Potentiation of Opioid Analgesia in Rats With Peripheral Neuropathy.Front Immunol. 2020 Dec 21;11:615327. doi: 10.3389/fimmu.2020.615327. eCollection 2020. Front Immunol. 2020. PMID: 33408720 Free PMC article.
-
Comparison of the beneficial effects of RS504393, maraviroc and cenicriviroc on neuropathic pain-related symptoms in rodents: behavioral and biochemical analyses.Int Immunopharmacol. 2020 Jul;84:106540. doi: 10.1016/j.intimp.2020.106540. Epub 2020 May 11. Int Immunopharmacol. 2020. PMID: 32402949
-
CCR2/CCR5 antagonist cenicriviroc reduces colonic inflammation and fibrosis in experimental colitis.J Gastroenterol Hepatol. 2024 Aug;39(8):1597-1605. doi: 10.1111/jgh.16622. Epub 2024 May 14. J Gastroenterol Hepatol. 2024. PMID: 38744472
-
Rationale of using the dual chemokine receptor CCR2/CCR5 inhibitor cenicriviroc for the treatment of COVID-19.PLoS Pathog. 2022 Jun 24;18(6):e1010547. doi: 10.1371/journal.ppat.1010547. eCollection 2022 Jun. PLoS Pathog. 2022. PMID: 35749425 Free PMC article. Review.
Cited by
-
A Missing Puzzle in Preclinical Studies-Are CCR2, CCR5, and Their Ligands' Roles Similar in Obesity-Induced Hypersensitivity and Diabetic Neuropathy?-Evidence from Rodent Models and Clinical Studies.Int J Mol Sci. 2024 Oct 21;25(20):11323. doi: 10.3390/ijms252011323. Int J Mol Sci. 2024. PMID: 39457105 Free PMC article. Review.
References
-
- Hansen C.S., Määttä L.L., Andersen S.T., Charles M.H. Diabetic Neuropathy. Contemporary Diabetes. Humana; Cham, Switzerland: 2023. The Epidemiology of Diabetic Neuropathy; pp. 5–36. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

