Production of Spinocerebellar Ataxia Type 3 Model Mice by Intravenous Injection of AAV-PHP.B Vectors
- PMID: 39000316
- PMCID: PMC11241190
- DOI: 10.3390/ijms25137205
Production of Spinocerebellar Ataxia Type 3 Model Mice by Intravenous Injection of AAV-PHP.B Vectors
Abstract
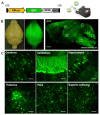
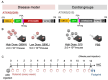
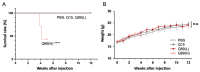
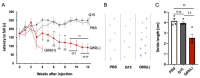
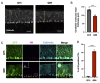
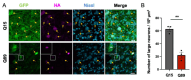
We aimed to produce a mouse model of spinocerebellar ataxia type 3 (SCA3) using the mouse blood-brain barrier (BBB)-penetrating adeno-associated virus (AAV)-PHP.B. Four-to-five-week-old C57BL/6 mice received injections of high-dose (2.0 × 1011 vg/mouse) or low-dose (5.0 × 1010 vg/mouse) AAV-PHP.B encoding a SCA3 causative gene containing abnormally long 89 CAG repeats [ATXN3(Q89)] under the control of the ubiquitous chicken β-actin hybrid (CBh) promoter. Control mice received high doses of AAV-PHP.B encoding ATXN3 with non-pathogenic 15 CAG repeats [ATXN3(Q15)] or phosphate-buffered saline (PBS) alone. More than half of the mice injected with high doses of AAV-PHP.B encoding ATXN3(Q89) died within 4 weeks after the injection. No mice in other groups died during the 12-week observation period. Mice injected with low doses of AAV-PHP.B encoding ATXN3(Q89) exhibited progressive motor uncoordination starting 4 weeks and a shorter stride in footprint analysis performed at 12 weeks post-AAV injection. Immunohistochemistry showed thinning of the molecular layer and the formation of nuclear inclusions in Purkinje cells from mice injected with low doses of AAV-PHP.B encoding ATXN3(Q89). Moreover, ATXN3(Q89) expression significantly reduced the number of large projection neurons in the cerebellar nuclei to one third of that observed in mice expressing ATXN3(Q15). This AAV-based approach is superior to conventional methods in that the required number of model mice can be created simply by injecting AAV, and the expression levels of the responsible gene can be adjusted by changing the amount of AAV injected. Moreover, this method may be applied to produce SCA3 models in non-human primates.
Keywords: AAV; AAV-PHP.B; Machado–Joseph disease; Purkinje cell; SCA3; Spinocerebellar ataxia type 3; adeno-associated virus; nuclear inclusion bodies.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Pharmacological enhancement of retinoid-related orphan receptor α function mitigates spinocerebellar ataxia type 3 pathology.Neurobiol Dis. 2019 Jan;121:263-273. doi: 10.1016/j.nbd.2018.10.014. Epub 2018 Oct 19. Neurobiol Dis. 2019. PMID: 30343032
-
miRNA-Mediated Knockdown of ATXN3 Alleviates Molecular Disease Hallmarks in a Mouse Model for Spinocerebellar Ataxia Type 3.Nucleic Acid Ther. 2022 Jun;32(3):194-205. doi: 10.1089/nat.2021.0020. Epub 2021 Dec 7. Nucleic Acid Ther. 2022. PMID: 34878314 Free PMC article.
-
Comparison of spinocerebellar ataxia type 3 mouse models identifies early gain-of-function, cell-autonomous transcriptional changes in oligodendrocytes.Hum Mol Genet. 2017 Sep 1;26(17):3362-3374. doi: 10.1093/hmg/ddx224. Hum Mol Genet. 2017. PMID: 28854700 Free PMC article.
-
Gene editing as a therapeutic strategy for spinocerebellar ataxia type-3.Rev Neurol (Paris). 2024 May;180(5):378-382. doi: 10.1016/j.neurol.2024.03.003. Epub 2024 Apr 4. Rev Neurol (Paris). 2024. PMID: 38580500 Review.
-
ATXN3: a multifunctional protein involved in the polyglutamine disease spinocerebellar ataxia type 3.Expert Rev Mol Med. 2024 Sep 25;26:e19. doi: 10.1017/erm.2024.10. Expert Rev Mol Med. 2024. PMID: 39320846 Free PMC article. Review.
Cited by
-
Optimal different adeno-associated virus capsid/promoter combinations to target specific cell types in the common marmoset cerebral cortex.Mol Ther Methods Clin Dev. 2024 Sep 13;32(4):101337. doi: 10.1016/j.omtm.2024.101337. eCollection 2024 Dec 12. Mol Ther Methods Clin Dev. 2024. PMID: 39391837 Free PMC article.
References
-
- Ren H., Hao Z., Wang G. Autophagy and Polyglutamine Disease. Adv. Exp. Med. Biol. 2020;1207:149–161. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

