High-Fat Diet Augments Myocardial Inflammation and Cardiac Dysfunction in Arrhythmogenic Cardiomyopathy
- PMID: 38999835
- PMCID: PMC11243382
- DOI: 10.3390/nu16132087
High-Fat Diet Augments Myocardial Inflammation and Cardiac Dysfunction in Arrhythmogenic Cardiomyopathy
Abstract
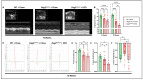
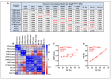
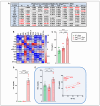
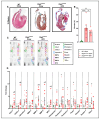
Arrhythmogenic cardiomyopathy (ACM) is a familial heart disease characterized by cardiac dysfunction, arrhythmias, and myocardial inflammation. Exercise and stress can influence the disease's progression. Thus, an investigation of whether a high-fat diet (HFD) contributes to ACM pathogenesis is warranted. In a robust ACM mouse model, 8-week-old Desmoglein-2 mutant (Dsg2mut/mut) mice were fed either an HFD or rodent chow for 8 weeks. Chow-fed wildtype (WT) mice served as controls. Echo- and electrocardiography images pre- and post-dietary intervention were obtained, and the lipid burden, inflammatory markers, and myocardial fibrosis were assessed at the study endpoint. HFD-fed Dsg2mut/mut mice showed numerous P-wave perturbations, reduced R-amplitude, left ventricle (LV) remodeling, and reduced ejection fraction (%LVEF). Notable elevations in plasma high-density lipoprotein (HDL) were observed, which correlated with the %LVEF. The myocardial inflammatory adipokines, adiponectin (AdipoQ) and fibroblast growth factor-1, were substantially elevated in HFD-fed Dsg2mut/mut mice, albeit no compounding effect was observed in cardiac fibrosis. The HFD not only potentiated cardiac dysfunction but additionally promoted adverse cardiac remodeling. Further investigation is warranted, particularly given elevated AdipoQ levels and the positive correlation of HDL with the %LVEF, which may suggest a protective effect. Altogether, the HFD worsened some, but not all, disease phenotypes in Dsg2mut/mut mice. Notwithstanding, diet may be a modifiable environmental factor in ACM disease progression.
Keywords: Desmoglein-2; arrhythmogenic cardiomyopathy; high-fat diet; lipids; myocardium.
Conflict of interest statement
S.P.C. is on the advisory board for Rejuvenate Bio and Who We Play For. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Activation of PPARα Ameliorates Cardiac Fibrosis in Dsg2-Deficient Arrhythmogenic Cardiomyopathy.Cells. 2022 Oct 11;11(20):3184. doi: 10.3390/cells11203184. Cells. 2022. PMID: 36291052 Free PMC article.
-
Efficacy and Safety of Angiotensin Receptor Blockers in a Pre-Clinical Model of Arrhythmogenic Cardiomyopathy.Int J Mol Sci. 2022 Nov 11;23(22):13909. doi: 10.3390/ijms232213909. Int J Mol Sci. 2022. PMID: 36430389 Free PMC article.
-
Therapeutic Modulation of the Immune Response in Arrhythmogenic Cardiomyopathy.Circulation. 2019 Oct 29;140(18):1491-1505. doi: 10.1161/CIRCULATIONAHA.119.040676. Epub 2019 Sep 19. Circulation. 2019. PMID: 31533459 Free PMC article.
-
Molecular insight into arrhythmogenic cardiomyopathy caused by DSG2 mutations.Biomed Pharmacother. 2023 Nov;167:115448. doi: 10.1016/j.biopha.2023.115448. Epub 2023 Sep 9. Biomed Pharmacother. 2023. PMID: 37696084 Review.
-
Special Article - Exercise-induced right ventricular injury or arrhythmogenic cardiomyopathy (ACM): The bright side and the dark side of the moon.Prog Cardiovasc Dis. 2020 Sep-Oct;63(5):671-681. doi: 10.1016/j.pcad.2020.03.015. Epub 2020 Mar 26. Prog Cardiovasc Dis. 2020. PMID: 32224113 Review.
References
-
- McKenna W.J., Thiene G., Nava A., Fontaliran F., Blomstrom-Lundqvist C., Fontaine G., Camerini F. Diagnosis of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Task Force of the Working Group Myocardial and Pericardial Disease of the European Society of Cardiology and of the Scientific Council on Cardiomyopathies of the International Society and Federation of Cardiology. Br. Heart J. 1994;71:215–218. doi: 10.1136/hrt.71.3.215. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

