Biological Activities of Novel Oleanolic Acid Derivatives from Bioconversion and Semi-Synthesis
- PMID: 38999041
- PMCID: PMC11243203
- DOI: 10.3390/molecules29133091
Biological Activities of Novel Oleanolic Acid Derivatives from Bioconversion and Semi-Synthesis
Abstract
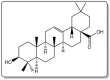
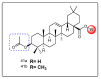
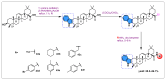
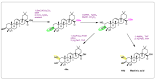
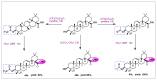
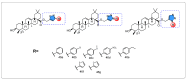
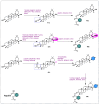
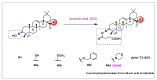
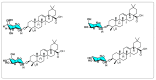
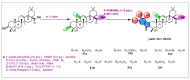
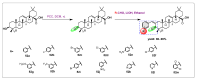
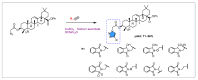
Oleanolic acid (OA) is a vegetable chemical that is present naturally in a number of edible and medicinal botanicals. It has been extensively studied by medicinal chemists and scientific researchers due to its biological activity against a wide range of diseases. A significant number of researchers have synthesized a variety of analogues of OA by modifying its structure with the intention of creating more potent biological agents and improving its pharmaceutical properties. In recent years, chemical and enzymatic techniques have been employed extensively to investigate and modify the chemical structure of OA. This review presents recent advancements in medical chemistry for the structural modification of OA, with a special focus on the biotransformation, semi-synthesis and relationship between the modified structures and their biopharmaceutical properties.
Keywords: anticancer activity; biological activities; biotransformation; medicinal chemistry; oleanolic acid; pharmaceutical properties; triterpenoids.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


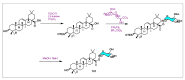
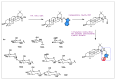
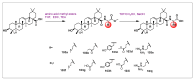
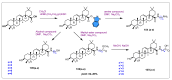
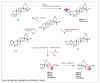
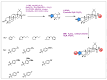
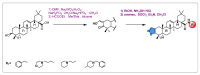
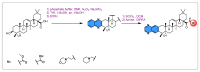
Similar articles
-
Recent advances in medicinal chemistry of oleanolic acid derivatives.Phytochemistry. 2022 Nov;203:113397. doi: 10.1016/j.phytochem.2022.113397. Epub 2022 Aug 25. Phytochemistry. 2022. PMID: 36029846 Review.
-
Oleanolic Acid and Its Derivatives: Biological Activities and Therapeutic Potential in Chronic Diseases.Molecules. 2017 Nov 13;22(11):1915. doi: 10.3390/molecules22111915. Molecules. 2017. PMID: 29137205 Free PMC article. Review.
-
Design, Synthesis and Evaluation of Pentacyclic Triterpenoids Similar to Glycyrrhetinic Acid Via Combination of Chemical and Microbial Modification as Glycogen Phosphorylases Inhibitor.J Microbiol Biotechnol. 2018 Nov 28;28(11):1876-1882. doi: 10.4014/jmb.1804.04042. J Microbiol Biotechnol. 2018. PMID: 30562883
-
Synthesis of Oleanolic Acid Analogues and Their Cytotoxic Effects on 3T3 Cell Line.Med Chem. 2018;14(6):617-625. doi: 10.2174/1573406414666180222094544. Med Chem. 2018. PMID: 29473521
-
Biotransformation of Oleanane and Ursane Triterpenic Acids.Molecules. 2020 Nov 25;25(23):5526. doi: 10.3390/molecules25235526. Molecules. 2020. PMID: 33255782 Free PMC article. Review.
References
-
- Yang Y., Chen K., Wang G., Liu H., Shao L., Zhou X., Liu L., Yang S. Discovery of Novel Pentacyclic Triterpene Acid Amide Derivatives as Excellent Antimicrobial Agents Dependent on Generation of Reactive Oxygen Species. Int. J. Mol. Sci. 2023;24:10566. doi: 10.3390/ijms241310566. - DOI - PMC - PubMed
-
- Sinda P.V.K., Tchuenguem R.T., Ponou B.K., Kühlborn J., Kianfé B.Y., Dzoyem J.P., Teponno R.B., Opatz T., Barboni L., Tapondjou L.A. Antimicrobial Activities of Extract, Fractions and Compounds from the Medicinal Plant Helichrysum odoratissimun (L.) Sweet (Asteraceae) S. Afr. J. Bot. 2022;147:937–941. doi: 10.1016/j.sajb.2022.03.049. - DOI
-
- Martial D.E., Dimitry M.Y., Selestin S.D., Nicolas N.Y. Hypolipidemic and Antioxidant Activity of Aqueous Extract of Clerodendrum thomsoniae Linn. (Verbenaceae) Leaves in Albino Rats, Rattus norvegicus (Muridae) J. Pharmacogn. Phytochem. 2020;9:595–602.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

