The differentiation of Lgr5+ progenitor cells on nanostructures of self-assembled silica beads
- PMID: 38995923
- PMCID: PMC11244819
- DOI: 10.1371/journal.pone.0304809
The differentiation of Lgr5+ progenitor cells on nanostructures of self-assembled silica beads
Abstract
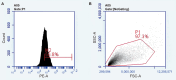
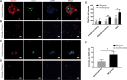
Supporting cells(SCs) have been demonstrated to be a reliable source for regenerating hair cells(HCs). Previous research has reported that Lgr5+ SCs can regenerate HCs both in vitro and in vivo. However, there is limited knowledge about the impact of the material on Lgr5+ cells. In this study, Lgr5+ cells were isolated from neonatal Lgr5-EGFP-CreERT2 transgenic mice by flow cytometry and then plated on self-assembled silica beads (SB). Lgr5+ cell differentiation was observed by immunofluorescence. We found that in the direct differentiation assay, the SB group generated more hair cells than the control group(*p < 0.05). Especially in the SB group, Lgr5+ progenitors generated significantly more Myo7a+ HCs outside of the colony than in the control group(**p < 0.01). In the sphere differentiation assay, we found that the diameter of spheres in the SB group was significantly larger compared to those of the control group(**p < 0.01). However, the difference in the ratio of myo7a+ cell counts was not obvious(P>0.05). The experiment proved that the self-assembled silica beads could promote the differentiation of Lgr5+ progenitors in vitro. Our findings implicate that nanostructures of self-assembled silica beads can be used as vectors for stem cell research in the inner ear.
Copyright: © 2024 Cai et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Wnt signaling induces proliferation of sensory precursors in the postnatal mouse cochlea.Proc Natl Acad Sci U S A. 2012 May 22;109(21):8167-72. doi: 10.1073/pnas.1202774109. Epub 2012 May 4. Proc Natl Acad Sci U S A. 2012. PMID: 22562792 Free PMC article.
-
Knockdown of Foxg1 in supporting cells increases the trans-differentiation of supporting cells into hair cells in the neonatal mouse cochlea.Cell Mol Life Sci. 2020 Apr;77(7):1401-1419. doi: 10.1007/s00018-019-03291-2. Epub 2019 Sep 4. Cell Mol Life Sci. 2020. PMID: 31485717 Free PMC article.
-
Characterization of the Transcriptomes of Lgr5+ Hair Cell Progenitors and Lgr5- Supporting Cells in the Mouse Cochlea.Front Mol Neurosci. 2017 Apr 26;10:122. doi: 10.3389/fnmol.2017.00122. eCollection 2017. Front Mol Neurosci. 2017. PMID: 28491023 Free PMC article.
-
Characterization of Lgr5+ progenitor cell transcriptomes in the apical and basal turns of the mouse cochlea.Oncotarget. 2016 Jul 5;7(27):41123-41141. doi: 10.18632/oncotarget.8636. Oncotarget. 2016. PMID: 27070092 Free PMC article.
-
Frizzled-9+ Supporting Cells Are Progenitors for the Generation of Hair Cells in the Postnatal Mouse Cochlea.Front Mol Neurosci. 2019 Jul 31;12:184. doi: 10.3389/fnmol.2019.00184. eCollection 2019. Front Mol Neurosci. 2019. PMID: 31427926 Free PMC article.
References
-
- Cox BC, Chai R, Lenoir A, Liu Z, Zhang L, Nguyen DH, et al.. Spontaneous hair cell regeneration in the neonatal mouse cochlea in vivo [published correction appears in Development. 2014. Apr;141(7):1599. Rubel, Edwin W [added]]. Development. 2014;141(4):816–829. doi: 10.1242/dev.103036 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

