Spatio-Temporal Regulation of Notch Activation in Asymmetrically Dividing Sensory Organ Precursor Cells in Drosophila melanogaster Epithelium
- PMID: 38994985
- PMCID: PMC11240559
- DOI: 10.3390/cells13131133
Spatio-Temporal Regulation of Notch Activation in Asymmetrically Dividing Sensory Organ Precursor Cells in Drosophila melanogaster Epithelium
Abstract
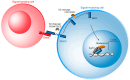
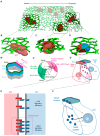
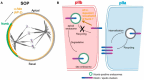
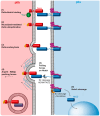
The Notch communication pathway, discovered in Drosophila over 100 years ago, regulates a wide range of intra-lineage decisions in metazoans. The division of the Drosophila mechanosensory organ precursor is the archetype of asymmetric cell division in which differential Notch activation takes place at cytokinesis. Here, we review the molecular mechanisms by which epithelial cell polarity, cell cycle and intracellular trafficking participate in controlling the directionality, subcellular localization and temporality of mechanosensitive Notch receptor activation in cytokinesis.
Keywords: Drosophila melanogaster; Notch signaling; asymmetric cell division; cell fate determinants; cell polarity; epithelial cells.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Par3 cooperates with Sanpodo for the assembly of Notch clusters following asymmetric division of Drosophila sensory organ precursor cells.Elife. 2021 Oct 1;10:e66659. doi: 10.7554/eLife.66659. Elife. 2021. PMID: 34596529 Free PMC article.
-
Modeling polarity buildup and cell fate decision in the fly eye: insight into the connection between the PCP and Notch pathways.Dev Genes Evol. 2008 Aug;218(8):413-26. doi: 10.1007/s00427-008-0235-y. Epub 2008 Jul 24. Dev Genes Evol. 2008. PMID: 18651172
-
Rabex-5 E3 and Rab5 GEF domains differ in their regulation of Ras, Notch, and PI3K signaling in Drosophila wing development.PLoS One. 2024 Oct 28;19(10):e0312274. doi: 10.1371/journal.pone.0312274. eCollection 2024. PLoS One. 2024. PMID: 39466792 Free PMC article.
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Notch signaling in prostate cancer: refining a therapeutic opportunity.Histol Histopathol. 2016 Feb;31(2):149-57. doi: 10.14670/HH-11-685. Epub 2015 Nov 2. Histol Histopathol. 2016. PMID: 26521657 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

