Targeting drug resistance in glioblastoma (Review)
- PMID: 38994761
- PMCID: PMC11251740
- DOI: 10.3892/ijo.2024.5668
Targeting drug resistance in glioblastoma (Review)
Abstract
Glioblastoma (GBM) is the most common malignancy of the central nervous system in adults. The current standard of care includes surgery, radiation therapy, temozolomide; and tumor‑treating fields leads to dismal overall survival. There are far limited treatments upon recurrence. Therapies to date are ineffective as a result of several factors, including the presence of the blood‑brain barrier, blood tumor barrier, glioma stem‑like cells and genetic heterogeneity in GBM. In the present review, the potential mechanisms that lead to treatment resistance in GBM and the measures which have been taken so far to attempt to overcome the resistance were discussed. The complex biology of GBM and lack of comprehensive understanding of the development of therapeutic resistance in GBM demands discovery of novel antigens that are targetable and provide effective therapeutic strategies.
Keywords: genetic heterogeneity; glioblastoma; radiation therapy; recurrence; resistance; therapeutics.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
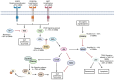
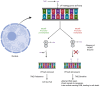
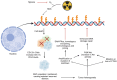
Similar articles
-
Targeted nanocomplex carrying siRNA against MALAT1 sensitizes glioblastoma to temozolomide.Nucleic Acids Res. 2018 Feb 16;46(3):1424-1440. doi: 10.1093/nar/gkx1221. Nucleic Acids Res. 2018. PMID: 29202181 Free PMC article.
-
A Multistep In Silico Approach Identifies Potential Glioblastoma Drug Candidates via Inclusive Molecular Targeting of Glioblastoma Stem Cells.Mol Neurobiol. 2024 Nov;61(11):9253-9271. doi: 10.1007/s12035-024-04139-y. Epub 2024 Apr 15. Mol Neurobiol. 2024. PMID: 38619743
-
Hitting a Moving Target: Glioma Stem Cells Demand New Approaches in Glioblastoma Therapy.Curr Cancer Drug Targets. 2017;17(3):236-254. doi: 10.2174/1568009616666161215161924. Curr Cancer Drug Targets. 2017. PMID: 27993114 Review.
-
Therapeutic strategies targeting glioblastoma stem cells.Recent Pat Anticancer Drug Discov. 2013 Sep;8(3):216-27. doi: 10.2174/15748928113089990002. Recent Pat Anticancer Drug Discov. 2013. PMID: 23607282 Review.
-
The histone demethylase KDM5A is a key factor for the resistance to temozolomide in glioblastoma.Cell Cycle. 2015;14(21):3418-29. doi: 10.1080/15384101.2015.1090063. Cell Cycle. 2015. PMID: 26566863 Free PMC article.
Cited by
-
Recent Treatment Strategies and Molecular Pathways in Resistance Mechanisms of Antiangiogenic Therapies in Glioblastoma.Cancers (Basel). 2024 Aug 27;16(17):2975. doi: 10.3390/cancers16172975. Cancers (Basel). 2024. PMID: 39272834 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

