Fueling CARs: metabolic strategies to enhance CAR T-cell therapy
- PMID: 38987856
- PMCID: PMC11238373
- DOI: 10.1186/s40164-024-00535-1
Fueling CARs: metabolic strategies to enhance CAR T-cell therapy
Abstract
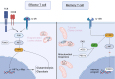
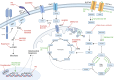
CAR T cells are widely applied for relapsed hematological cancer patients. With six approved cell therapies, for Multiple Myeloma and other B-cell malignancies, new insights emerge. Profound evidence shows that patients who fail CAR T-cell therapy have, aside from antigen escape, a more glycolytic and weakened metabolism in their CAR T cells, accompanied by a short lifespan. Recent advances show that CAR T cells can be metabolically engineered towards oxidative phosphorylation, which increases their longevity via epigenetic and phenotypical changes. In this review we elucidate various strategies to rewire their metabolism, including the design of the CAR construct, co-stimulus choice, genetic modifications of metabolic genes, and pharmacological interventions. We discuss their potential to enhance CAR T-cell functioning and persistence through memory imprinting, thereby improving outcomes. Furthermore, we link the pharmacological treatments with their anti-cancer properties in hematological malignancies to ultimately suggest novel combination strategies.
Keywords: CAR T cells; Co-stimulus; Drug repurposing; Metabolism; Mitochondria.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Challenges and Prospects of Chimeric Antigen Receptor T-cell Therapy for Metastatic Prostate Cancer.Eur Urol. 2020 Mar;77(3):299-308. doi: 10.1016/j.eururo.2019.08.014. Epub 2019 Aug 28. Eur Urol. 2020. PMID: 31471138 Review.
-
Paediatric Strategy Forum for medicinal product development of chimeric antigen receptor T-cells in children and adolescents with cancer: ACCELERATE in collaboration with the European Medicines Agency with participation of the Food and Drug Administration.Eur J Cancer. 2022 Jan;160:112-133. doi: 10.1016/j.ejca.2021.10.016. Epub 2021 Nov 25. Eur J Cancer. 2022. PMID: 34840026 Review.
-
A metabolic switch to memory CAR T cells: Implications for cancer treatment.Cancer Lett. 2021 Mar 1;500:107-118. doi: 10.1016/j.canlet.2020.12.004. Epub 2020 Dec 5. Cancer Lett. 2021. PMID: 33290868
-
Importance of T, NK, CAR T and CAR NK Cell Metabolic Fitness for Effective Anti-Cancer Therapy: A Continuous Learning Process Allowing the Optimization of T, NK and CAR-Based Anti-Cancer Therapies.Cancers (Basel). 2021 Dec 30;14(1):183. doi: 10.3390/cancers14010183. Cancers (Basel). 2021. PMID: 35008348 Free PMC article. Review.
-
Custom CARs: Leveraging the Adaptability of Allogeneic CAR Therapies to Address Current Challenges in Relapsed/Refractory DLBCL.Front Immunol. 2022 May 18;13:887866. doi: 10.3389/fimmu.2022.887866. eCollection 2022. Front Immunol. 2022. PMID: 35663947 Free PMC article. Review.
Cited by
-
Optimizing CD8+ T cell-based immunotherapy via metabolic interventions: a comprehensive review of intrinsic and extrinsic modulators.Exp Hematol Oncol. 2024 Oct 22;13(1):103. doi: 10.1186/s40164-024-00575-7. Exp Hematol Oncol. 2024. PMID: 39438986 Free PMC article. Review.
-
Therapeutic targets of armored chimeric antigen receptor T cells navigating the tumor microenvironment.Exp Hematol Oncol. 2024 Sep 30;13(1):96. doi: 10.1186/s40164-024-00564-w. Exp Hematol Oncol. 2024. PMID: 39350256 Free PMC article. Review.
References
-
- Dai H, Zhu C, Huai Q, Xu W, Zhu J, Zhang X et al. Chimeric antigen receptor-modified macrophages ameliorate liver fibrosis in preclinical models. J Hepatol. 2024. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

