Ragopathies and the rising influence of RagGTPases on human diseases
- PMID: 38987251
- PMCID: PMC11237164
- DOI: 10.1038/s41467-024-50034-4
Ragopathies and the rising influence of RagGTPases on human diseases
Abstract
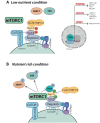
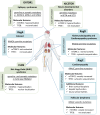
RagGTPases (Rags) play an essential role in the regulation of cell metabolism by controlling the activities of both mechanistic target of rapamycin complex 1 (mTORC1) and Transcription factor EB (TFEB). Several diseases, herein named ragopathies, are associated to Rags dysfunction. These diseases may be caused by mutations either in genes encoding the Rags, or in their upstream regulators. The resulting phenotypes may encompass a variety of clinical features such as cataract, kidney tubulopathy, dilated cardiomyopathy and several types of cancer. In this review, we focus on the key clinical, molecular and physio-pathological features of ragopathies, aiming to shed light on their underlying mechanisms.
© 2024. The Author(s).
Conflict of interest statement
A.B. is cofounder of CASMA Therapeutics, Inc, and Advisory board member of Avilar and Amplify Therapeutics. The remaining authors declare no competing interests.
Figures
Similar articles
-
RagD auto-activating mutations impair MiT/TFE activity in kidney tubulopathy and cardiomyopathy syndrome.Nat Commun. 2023 May 15;14(1):2775. doi: 10.1038/s41467-023-38428-2. Nat Commun. 2023. PMID: 37188688 Free PMC article.
-
Past, present, and future perspectives of transcription factor EB (TFEB): mechanisms of regulation and association with disease.Cell Death Differ. 2022 Aug;29(8):1433-1449. doi: 10.1038/s41418-022-01028-6. Epub 2022 Jun 23. Cell Death Differ. 2022. PMID: 35739255 Free PMC article. Review.
-
TFEB Overexpression, Not mTOR Inhibition, Ameliorates RagCS75Y Cardiomyopathy.Int J Mol Sci. 2021 May 23;22(11):5494. doi: 10.3390/ijms22115494. Int J Mol Sci. 2021. PMID: 34071043 Free PMC article.
-
TFEB-driven endocytosis coordinates MTORC1 signaling and autophagy.Autophagy. 2019 Jan;15(1):151-164. doi: 10.1080/15548627.2018.1511504. Epub 2018 Sep 10. Autophagy. 2019. PMID: 30145926 Free PMC article.
-
TFEB: a double-edged sword for tumor metastasis.J Mol Med (Berl). 2023 Aug;101(8):917-929. doi: 10.1007/s00109-023-02337-0. Epub 2023 Jun 17. J Mol Med (Berl). 2023. PMID: 37328669 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA260205/CA/NCI NIH HHS/United States
- IG-22103/Associazione Italiana per la Ricerca sul Cancro (Italian Association for Cancer Research)
- LYSOSOMICS 694282/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Priority Excellent Science | H2020 European Research Council (H2020 Excellent Science - European Research Council)
- PRIN 202032AZT3/Ministero dell'Istruzione, dell'Università e della Ricerca (Ministry of Education, University and Research)
LinkOut - more resources
Full Text Sources

