Endometriotic Tissue-derived Exosomes Downregulate NKG2D-mediated Cytotoxicity and Promote Apoptosis: Mechanisms for Survival of Ectopic Endometrial Tissue in Endometriosis
- PMID: 38984872
- PMCID: PMC11335327
- DOI: 10.4049/jimmunol.2300781
Endometriotic Tissue-derived Exosomes Downregulate NKG2D-mediated Cytotoxicity and Promote Apoptosis: Mechanisms for Survival of Ectopic Endometrial Tissue in Endometriosis
Abstract
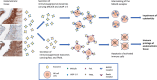
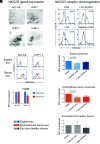
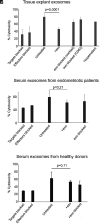
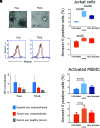
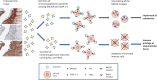
Endometriosis, affecting 10% of women, is defined as implantation, survival, and growth of endometrium-like/endometriotic tissue outside the uterine cavity, causing inflammation, infertility, pain, and susceptibility to ovarian cancer. Despite extensive studies, its etiology and pathogenesis are poorly understood and largely unknown. The prevailing view is that the immune system of endometriosis patients fails to clear ectopically disseminated endometrium from retrograde menstruation. Exosomes are small extracellular vesicles that exhibit immunomodulatory properties. We studied the role of endometriotic tissue-secreted exosomes in the pathophysiology of endometriosis. Two exosome-mediated mechanisms known to impair the immune response were investigated: 1) downregulation of NKG2D-mediated cytotoxicity and 2) FasL- and TRAIL-induced apoptosis of activated immune cells. We showed that secreted endometriotic exosomes isolated from supernatants of short-term explant cultures carry the NKG2D ligands MICA/B and ULBP1-3 and the proapoptotic molecules FasL and TRAIL on their surface, i.e., signature molecules of exosome-mediated immune suppression. Acting as decoys, these exosomes downregulate the NKG2D receptor, impair NKG2D-mediated cytotoxicity, and induce apoptosis of activated PBMCs and Jurkat cells through the FasL- and TRAIL pathway. The secreted endometriotic exosomes create an immunosuppressive gradient at the ectopic site, forming a "protective shield" around the endometriotic lesions. This gradient guards the endometriotic lesions against clearance by a cytotoxic attack and creates immunologic privilege by induction of apoptosis in activated immune cells. Taken together, our results provide a plausible, exosome-based mechanistic explanation for the immune dysfunction and the compromised immune surveillance in endometriosis and contribute novel insights into the pathogenesis of this enigmatic disease.
Copyright © 2024 by The American Association of Immunologists, Inc.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures

Similar articles
-
Exosomes secreted by human placenta carry functional Fas ligand and TRAIL molecules and convey apoptosis in activated immune cells, suggesting exosome-mediated immune privilege of the fetus.J Immunol. 2013 Dec 1;191(11):5515-23. doi: 10.4049/jimmunol.1301885. Epub 2013 Nov 1. J Immunol. 2013. PMID: 24184557
-
Thermal- and oxidative stress causes enhanced release of NKG2D ligand-bearing immunosuppressive exosomes in leukemia/lymphoma T and B cells.PLoS One. 2011 Feb 25;6(2):e16899. doi: 10.1371/journal.pone.0016899. PLoS One. 2011. PMID: 21364924 Free PMC article.
-
Platelet-derived TGF-β1 mediates the down-modulation of NKG2D expression and may be responsible for impaired natural killer (NK) cytotoxicity in women with endometriosis.Hum Reprod. 2016 Jul;31(7):1462-74. doi: 10.1093/humrep/dew057. Epub 2016 Apr 30. Hum Reprod. 2016. PMID: 27130956
-
Cancer exosomes and NKG2D receptor-ligand interactions: impairing NKG2D-mediated cytotoxicity and anti-tumour immune surveillance.Semin Cancer Biol. 2014 Oct;28:24-30. doi: 10.1016/j.semcancer.2014.02.010. Epub 2014 Mar 3. Semin Cancer Biol. 2014. PMID: 24602822 Review.
-
Generation of soluble NKG2D ligands: proteolytic cleavage, exosome secretion and functional implications.Scand J Immunol. 2013 Aug;78(2):120-9. doi: 10.1111/sji.12072. Scand J Immunol. 2013. PMID: 23679194 Review.
Cited by
-
Association of circulating cytokine levels and tissue-infiltrating myeloid cells with achalasia: results from Mendelian randomization and validation through clinical characteristics and single-cell RNA sequencing.J Gastroenterol. 2024 Dec;59(12):1079-1091. doi: 10.1007/s00535-024-02155-2. Epub 2024 Oct 8. J Gastroenterol. 2024. PMID: 39377966
References
-
- Zondervan, K. T., Becker C. M., Missmer S. A.. 2020. Endometriosis. N. Engl. J. Med. 382: 1244–1256. - PubMed
-
- Harada, T., Iwabe T., Terakawa N.. 2001. Role of cytokines in endometriosis. Fertil. Steril. 76: 1–10. - PubMed
-
- Cramer, D. W., Missmer S. A.. 2002. The epidemiology of endometriosis. Ann. N. Y. Acad. Sci. 955: 11–22. - PubMed
-
- Giudice, L. C., Kao L. C.. 2004. Endometriosis. Lancet 364: 1789–1799. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

