Neutrophil extracellular traps promote immunopathogenesis of virus-induced COPD exacerbations
- PMID: 38982052
- PMCID: PMC11233599
- DOI: 10.1038/s41467-024-50197-0
Neutrophil extracellular traps promote immunopathogenesis of virus-induced COPD exacerbations
Abstract
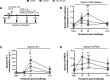
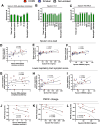
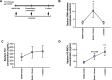
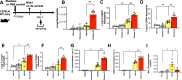
Respiratory viruses are a major trigger of exacerbations in chronic obstructive pulmonary disease (COPD). Airway neutrophilia is a hallmark feature of stable and exacerbated COPD but roles played by neutrophil extracellular traps (NETS) in driving disease pathogenesis are unclear. Here, using human studies of experimentally-induced and naturally-occurring exacerbations we identify that rhinovirus infection induces airway NET formation which is amplified in COPD and correlates with magnitude of inflammation and clinical exacerbation severity. We show that inhibiting NETosis protects mice from immunopathology in a model of virus-exacerbated COPD. NETs drive inflammation during exacerbations through release of double stranded DNA (dsDNA) and administration of DNAse in mice has similar protective effects. Thus, NETosis, through release of dsDNA, has a functional role in the pathogenesis of COPD exacerbations. These studies open up the potential for therapeutic targeting of NETs or dsDNA as a strategy for treating virus-exacerbated COPD.
© 2024. The Author(s).
Conflict of interest statement
S.L.J. has personally received consultancy fees from AstraZeneca, Bioforce, Enanta and GlaxoSmithKline. S.L.J. is an inventor on patents on the use of inhaled interferons for treatment of exacerbations of airway diseases and on rhinovirus vaccines. S.L.J. is Director and shareholder of Virtus Respiratory Research Ltd. JDC has received research grants from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Gilead Sciences, Grifols, Novartis, Insmed and Trudell; and received consultancy or speaker fees from Antabio, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Insmed, Janssen, Novartis, Pfizer, Trudell and Zambo. A.S. has received honoraria for speaking from AstraZeneca. A.D.A. is Chief Medical Officer at Santersus AG. The remaining authors declare no competing interests.
Figures




Similar articles
-
Host DNA released by NETosis promotes rhinovirus-induced type-2 allergic asthma exacerbation.Nat Med. 2017 Jun;23(6):681-691. doi: 10.1038/nm.4332. Epub 2017 May 1. Nat Med. 2017. PMID: 28459437 Free PMC article.
-
DNA of neutrophil extracellular traps promote NF-κB-dependent autoimmunity via cGAS/TLR9 in chronic obstructive pulmonary disease.Signal Transduct Target Ther. 2024 Jun 17;9(1):163. doi: 10.1038/s41392-024-01881-6. Signal Transduct Target Ther. 2024. PMID: 38880789 Free PMC article.
-
A human rhinovirus model of chronic obstructive pulmonary disease exacerbations.Contrib Microbiol. 2007;14:101-112. doi: 10.1159/000107057. Contrib Microbiol. 2007. PMID: 17684335 Review.
-
Antiviral immunity is impaired in COPD patients with frequent exacerbations.Am J Physiol Lung Cell Mol Physiol. 2019 Dec 1;317(6):L893-L903. doi: 10.1152/ajplung.00253.2019. Epub 2019 Sep 12. Am J Physiol Lung Cell Mol Physiol. 2019. PMID: 31513433 Free PMC article.
-
Human rhinovirus infection and COPD: role in exacerbations and potential for therapeutic targets.Expert Rev Respir Med. 2020 Aug;14(8):777-789. doi: 10.1080/17476348.2020.1764354. Epub 2020 Jun 4. Expert Rev Respir Med. 2020. PMID: 32498634 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

