Antitumour effects of SFX-01 molecule in combination with ionizing radiation in preclinical and in vivo models of rhabdomyosarcoma
- PMID: 38977944
- PMCID: PMC11229215
- DOI: 10.1186/s12885-024-12536-8
Antitumour effects of SFX-01 molecule in combination with ionizing radiation in preclinical and in vivo models of rhabdomyosarcoma
Abstract
Background: Despite a multimodal approach including surgery, chemo- and radiotherapy, the 5-year event-free survival rate for rhabdomyosarcoma (RMS), the most common soft tissue sarcoma in childhood, remains very poor for metastatic patients, mainly due to the selection and proliferation of tumour cells driving resistance mechanisms. Personalised medicine-based protocols using new drugs or targeted therapies in combination with conventional treatments have the potential to enhance the therapeutic effects, while minimizing damage to healthy tissues in a wide range of human malignancies, with several clinical trials being started. In this study, we analysed, for the first time, the antitumour activity of SFX-01, a complex of synthetic d, l-sulforaphane stabilised in alpha-cyclodextrin (Evgen Pharma plc, UK), used as single agent and in combination with irradiation, in four preclinical models of alveolar and embryonal RMS. Indeed, SFX-01 has shown promise in preclinical studies for its ability to modulate cellular pathways involved in inflammation and oxidative stress that are essential to be controlled in cancer treatment.
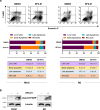
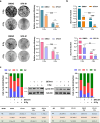
Methods: RH30, RH4 (alveolar RMS), RD and JR1 (embryonal RMS) cell lines as well as mouse xenograft models of RMS were used to evaluate the biological and molecular effects induced by SFX-01 treatment. Flow cytometry and the modulation of key markers analysed by q-PCR and Western blot were used to assess cell proliferation, apoptosis, autophagy and production of intracellular reactive oxygen species (ROS) in RMS cells exposed to SFX-01. The ability to migrate and invade was also investigated with specific assays. The possible synergistic effects between SFX-01 and ionising radiation (IR) was studied in both the in vitro and in vivo studies. Student's t-test or two-way ANOVA were used to test the statistical significance of two or more comparisons, respectively.
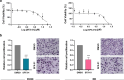
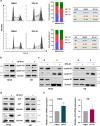
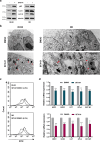
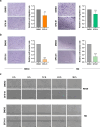
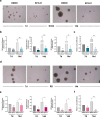
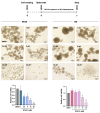
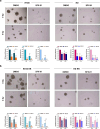
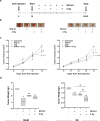
Results: SFX-01 treatment exhibited cytostatic and cytotoxic effects, mediated by G2 cell cycle arrest, apoptosis induction and suppression of autophagy. Moreover, SFX-01 was able to inhibit the formation and the proliferation of 3D tumorspheres as monotherapy and in combination with IR. Finally, SFX-01, when orally administered as single agent, displayed a pattern of efficacy at reducing the growth of tumour masses in RMS xenograft mouse models; when combined with a radiotherapy regime, it was observed to act synergistically, resulting in a more positive outcome than would be expected by adding each exposure alone.
Conclusions: In summary, our results provide evidence for the antitumour properties of SFX-01 in preclinical models of RMS tumours, both as a standalone treatment and in combination with irradiation. These forthcoming findings are crucial for deeper investigations of SFX-01 molecular mechanisms against RMS and for setting up clinical trials in RMS patients in order to use the SFX-01/IR co-treatment as a promising therapeutic approach, particularly in the clinical management of aggressive RMS disease.
Keywords: 3D tumorspheres; Cell cycle arrest; Oxidative stress; Radiotherapy; Rhabdomyosarcoma, SFX-01; Sulforaphane.
© 2024. The Author(s).
Conflict of interest statement
C.F. is a scientific advisor at Evgen Pharma. The other authors declare that they have no competing interests.
Figures

Similar articles
-
Crizotinib-induced antitumour activity in human alveolar rhabdomyosarcoma cells is not solely dependent on ALK and MET inhibition.J Exp Clin Cancer Res. 2015 Oct 6;34:112. doi: 10.1186/s13046-015-0228-4. J Exp Clin Cancer Res. 2015. PMID: 26445453 Free PMC article.
-
The anti-tumor growth effect of a novel agent DMAMCL in rhabdomyosarcoma in vitro and in vivo.J Exp Clin Cancer Res. 2019 Mar 8;38(1):118. doi: 10.1186/s13046-019-1107-1. J Exp Clin Cancer Res. 2019. PMID: 30850026 Free PMC article.
-
PARP inhibitors affect growth, survival and radiation susceptibility of human alveolar and embryonal rhabdomyosarcoma cell lines.J Cancer Res Clin Oncol. 2019 Jan;145(1):137-152. doi: 10.1007/s00432-018-2774-6. Epub 2018 Oct 24. J Cancer Res Clin Oncol. 2019. PMID: 30357520 Free PMC article.
-
Engaging Cell Death Pathways for the Treatment of Rhabdomyosarcoma.Crit Rev Oncog. 2016;21(3-4):221-239. doi: 10.1615/CritRevOncog.2016016996. Crit Rev Oncog. 2016. PMID: 27915973 Review.
-
Genetics, epigenetics and redox homeostasis in rhabdomyosarcoma: Emerging targets and therapeutics.Redox Biol. 2019 Jul;25:101124. doi: 10.1016/j.redox.2019.101124. Epub 2019 Jan 25. Redox Biol. 2019. PMID: 30709791 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

