A pharmacovigilance study of chronic kidney disease in diabetes mellitus patients with statin treatment by using the US Food and Drug Administration adverse event reporting system
- PMID: 38974040
- PMCID: PMC11224537
- DOI: 10.3389/fphar.2024.1363501
A pharmacovigilance study of chronic kidney disease in diabetes mellitus patients with statin treatment by using the US Food and Drug Administration adverse event reporting system
Abstract
Background: Statins were regarded as a main medication for managing hypercholesterolemia. Administration of statin therapy could reduce the incidence of cardiovascular disease in individuals diagnosed with type 2 diabetes mellitus (DM), which was recognized by multipal clinical guidelines. But previous studies had conflicting results on whether the long-term use of statins could benefit the renal function in diabetic patients.
Aim: To evaluate the association between statin treatment and Chronic Kidney Disease in DM patients.
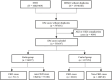
Methods: This is a retrospective disproportionality analysis and cohort study based on real-world data. All DM cases reported in US Food and Drug Administration adverse event reporting system (FAERS) between the first quarter of 2004 and the fourth quarter of 2022 were included. Disproportionality analyses were conducted by estimating the reporting odds ratio (ROR) and the information component (IC). We further compared the CKD odds ratio (OR) between the statins group and the other primary suspected drug group among the included diabetes mellitus cases.
Results: We finally included 593647 DM cases from FAERS, 5113 (5.31%) CKD cases in the statins group and 8810 (1.77%) CKD cases in the control group. Data analysis showed that the statins group showed a significant CKD signal (ROR: 3.11, 95% CI: 3.00-3.22; IC: 1.18, 95% CI: 1.07-1.29). In case group with two or more statins treatment history, the CKD signal was even stronger (ROR: 19.56, 95% CI: 18.10-21.13; IC: 3.70, 95% CI:3.44-3.93) compared with cases with one statin treatment history.
Conclusion: The impact of statin therapy on the progression of renal disease in individuals diagnosed with type 2 diabetes mellitus (DM) remains inconclusive. After data mining on the current FAERS dataset, we discovered significant signals between statin treatment and CKD in diabetic patients. Furthermore, the incidence rate of CKD was higher among DM patients who used statins compared to those who did not.
Keywords: FAERS; chronic kidney disease; diabetes mellitus; disproportionality analyses; statins.
Copyright © 2024 Zhang, Guo, Wei, Yan, Shan, Wu and Wu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Reporting of Drug-Induced Myopathies Associated with the Combination of Statins and Daptomycin: A Disproportionality Analysis Using the US Food and Drug Administration Adverse Event Reporting System.J Clin Med. 2023 May 18;12(10):3548. doi: 10.3390/jcm12103548. J Clin Med. 2023. PMID: 37240654 Free PMC article.
-
Pregnancy-related adverse events associated with statins: a real-world pharmacovigilance study of the FDA Adverse Event Reporting System (FAERS).Expert Opin Drug Saf. 2024 Mar;23(3):313-321. doi: 10.1080/14740338.2023.2251888. Epub 2023 Sep 1. Expert Opin Drug Saf. 2024. PMID: 37612600
-
Association between proton pump inhibitors and rhabdomyolysis risk: a post-marketing surveillance using FDA adverse event reporting system (FAERS) database.Ther Adv Drug Saf. 2023 Feb 27;14:20420986231154075. doi: 10.1177/20420986231154075. eCollection 2023. Ther Adv Drug Saf. 2023. PMID: 36875514 Free PMC article.
-
HMG CoA reductase inhibitors (statins) for people with chronic kidney disease not requiring dialysis.Cochrane Database Syst Rev. 2014 May 31;(5):CD007784. doi: 10.1002/14651858.CD007784.pub2. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2023 Nov 29;11:CD007784. doi: 10.1002/14651858.CD007784.pub3 PMID: 24880031 Updated. Review.
-
Lipid Screening in Childhood and Adolescence for Detection of Familial Hypercholesterolemia: A Systematic Evidence Review for the U.S. Preventive Services Task Force [Internet].Rockville (MD): Agency for Healthcare Research and Quality (US); 2016 Aug. Report No.: 14-05204-EF-2. Rockville (MD): Agency for Healthcare Research and Quality (US); 2016 Aug. Report No.: 14-05204-EF-2. PMID: 27559556 Free Books & Documents. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources