Rankl genetic deficiency and functional blockade undermine skeletal stem and progenitor cell differentiation
- PMID: 38971808
- PMCID: PMC11227705
- DOI: 10.1186/s13287-024-03803-3
Rankl genetic deficiency and functional blockade undermine skeletal stem and progenitor cell differentiation
Abstract
Background: Skeletal Stem Cells (SSCs) are required for skeletal development, homeostasis, and repair. The perspective of their wide application in regenerative medicine approaches has supported research in this field, even though so far results in the clinic have not reached expectations, possibly due also to partial knowledge of intrinsic, potentially actionable SSC regulatory factors. Among them, the pleiotropic cytokine RANKL, with essential roles also in bone biology, is a candidate deserving deep investigation.
Methods: To dissect the role of the RANKL cytokine in SSC biology, we performed ex vivo characterization of SSCs and downstream progenitors (SSPCs) in mice lacking Rankl (Rankl-/-) by means of cytofluorimetric sorting and analysis of SSC populations from different skeletal compartments, gene expression analysis, and in vitro osteogenic differentiation. In addition, we assessed the effect of the pharmacological treatment with the anti-RANKL blocking antibody Denosumab (approved for therapy in patients with pathological bone loss) on the osteogenic potential of bone marrow-derived stromal cells from human healthy subjects (hBMSCs).
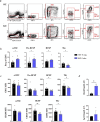
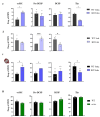
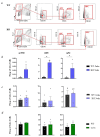
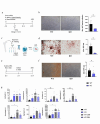
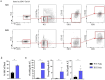
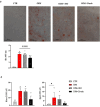
Results: We found that, regardless of the ossification type of bone, osteochondral SSCs had a higher frequency and impaired differentiation along the osteochondrogenic lineage in Rankl-/- mice as compared to wild-type. Rankl-/- mice also had increased frequency of committed osteochondrogenic and adipogenic progenitor cells deriving from perivascular SSCs. These changes were not due to the peculiar bone phenotype of increased density caused by lack of osteoclast resorption (defined osteopetrosis); indeed, they were not found in another osteopetrotic mouse model, i.e., the oc/oc mouse, and were therefore not due to osteopetrosis per se. In addition, Rankl-/- SSCs and primary osteoblasts showed reduced mineralization capacity. Of note, hBMSCs treated in vitro with Denosumab had reduced osteogenic capacity compared to control cultures.
Conclusions: We provide for the first time the characterization of SSPCs from mouse models of severe recessive osteopetrosis. We demonstrate that Rankl genetic deficiency in murine SSCs and functional blockade in hBMSCs reduce their osteogenic potential. Therefore, we propose that RANKL is an important regulatory factor of SSC features with translational relevance.
Keywords: Denosumab; Differentiation; Osteopetrosis; RANKL; Skeletal stem cells; Therapy.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Similar articles
-
Murine Rankl-/- Mesenchymal Stromal Cells Display an Osteogenic Differentiation Defect Improved by a RANKL-Expressing Lentiviral Vector.Stem Cells. 2017 May;35(5):1365-1377. doi: 10.1002/stem.2574. Epub 2017 Mar 1. Stem Cells. 2017. PMID: 28100034
-
Transglutaminases factor XIII-A and TG2 regulate resorption, adipogenesis and plasma fibronectin homeostasis in bone and bone marrow.Cell Death Differ. 2017 May;24(5):844-854. doi: 10.1038/cdd.2017.21. Epub 2017 Apr 7. Cell Death Differ. 2017. PMID: 28387755 Free PMC article.
-
Mesenchymal Stromal Cell-Seeded Biomimetic Scaffolds as a Factory of Soluble RANKL in Rankl-Deficient Osteopetrosis.Stem Cells Transl Med. 2019 Jan;8(1):22-34. doi: 10.1002/sctm.18-0085. Epub 2018 Sep 5. Stem Cells Transl Med. 2019. PMID: 30184340 Free PMC article.
-
Skeletal stem and progenitor cells in bone development and repair.J Bone Miner Res. 2024 Jul 23;39(6):633-654. doi: 10.1093/jbmr/zjae069. J Bone Miner Res. 2024. PMID: 38696703 Review.
-
Finding a Toll on the Route: The Fate of Osteoclast Progenitors After Toll-Like Receptor Activation.Front Immunol. 2019 Jul 17;10:1663. doi: 10.3389/fimmu.2019.01663. eCollection 2019. Front Immunol. 2019. PMID: 31379855 Free PMC article. Review.
Cited by
-
Adjuvant denosumab for early breast cancer-Evidence and controversy.Breast. 2024 Dec;78:103826. doi: 10.1016/j.breast.2024.103826. Epub 2024 Oct 23. Breast. 2024. PMID: 39509862 Free PMC article.
-
Promotion of Bone Formation in a Rat Osteoporotic Vertebral Body Defect Model via Suppression of Osteoclastogenesis by Ectopic Embryonic Calvaria Derived Mesenchymal Stem Cells.Int J Mol Sci. 2024 Jul 26;25(15):8174. doi: 10.3390/ijms25158174. Int J Mol Sci. 2024. PMID: 39125746 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

