The septin cytoskeleton is required for plasma membrane repair
- PMID: 38969946
- PMCID: PMC11387490
- DOI: 10.1038/s44319-024-00195-6
The septin cytoskeleton is required for plasma membrane repair
Abstract
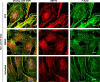
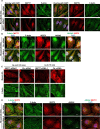
Plasma membrane repair is a fundamental homeostatic process of eukaryotic cells. Here, we report a new function for the conserved cytoskeletal proteins known as septins in the repair of cells perforated by pore-forming toxins or mechanical disruption. Using a silencing RNA screen, we identified known repair factors (e.g. annexin A2, ANXA2) and novel factors such as septin 7 (SEPT7) that is essential for septin assembly. Upon plasma membrane injury, the septin cytoskeleton is extensively redistributed to form submembranous domains arranged as knob and loop structures containing F-actin, myosin IIA, S100A11, and ANXA2. Formation of these domains is Ca2+-dependent and correlates with plasma membrane repair efficiency. Super-resolution microscopy revealed that septins and F-actin form intertwined filaments associated with ANXA2. Depletion of SEPT7 prevented ANXA2 recruitment and formation of submembranous actomyosin domains. However, ANXA2 depletion had no effect on domain formation. Collectively, our data support a novel septin-based mechanism for resealing damaged cells, in which the septin cytoskeleton plays a key structural role in remodeling the plasma membrane by promoting the formation of SEPT/F-actin/myosin IIA/ANXA2/S100A11 repair domains.
Keywords: Annexin A2; F-actin; Plasma Membrane Repair; Pore-forming Toxin; Septin.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Update of
-
The Septin Cytoskeleton is Required for Plasma Membrane Repair.bioRxiv [Preprint]. 2024 Jan 6:2023.07.12.548547. doi: 10.1101/2023.07.12.548547. bioRxiv. 2024. Update in: EMBO Rep. 2024 Sep;25(9):3870-3895. doi: 10.1038/s44319-024-00195-6 PMID: 37503091 Free PMC article. Updated. Preprint.
Similar articles
-
The Septin Cytoskeleton is Required for Plasma Membrane Repair.bioRxiv [Preprint]. 2024 Jan 6:2023.07.12.548547. doi: 10.1101/2023.07.12.548547. bioRxiv. 2024. Update in: EMBO Rep. 2024 Sep;25(9):3870-3895. doi: 10.1038/s44319-024-00195-6 PMID: 37503091 Free PMC article. Updated. Preprint.
-
Submembranous septins as relatively stable components of actin-based membrane skeleton.Cytoskeleton (Hoboken). 2011 Sep;68(9):512-25. doi: 10.1002/cm.20528. Epub 2011 Aug 25. Cytoskeleton (Hoboken). 2011. PMID: 21800439
-
Human septins organize as octamer-based filaments and mediate actin-membrane anchoring in cells.J Cell Biol. 2023 Mar 6;222(3):e202203016. doi: 10.1083/jcb.202203016. Epub 2022 Dec 23. J Cell Biol. 2023. PMID: 36562751 Free PMC article.
-
Septin and actin contributions to endothelial cell-cell junctions and monolayer integrity.Cytoskeleton (Hoboken). 2023 Jul-Aug;80(7-8):228-241. doi: 10.1002/cm.21732. Epub 2022 Oct 17. Cytoskeleton (Hoboken). 2023. PMID: 36205643 Free PMC article. Review.
-
The state of the septin cytoskeleton from assembly to function.Curr Opin Cell Biol. 2021 Feb;68:105-112. doi: 10.1016/j.ceb.2020.10.007. Epub 2020 Nov 11. Curr Opin Cell Biol. 2021. PMID: 33188984 Free PMC article. Review.
Cited by
-
Calpains Orchestrate Secretion of Annexin-containing Microvesicles during Membrane Repair.bioRxiv [Preprint]. 2024 Sep 6:2024.09.05.611512. doi: 10.1101/2024.09.05.611512. bioRxiv. 2024. PMID: 39282443 Free PMC article. Preprint.
References
-
- Atanassoff AP, Wolfmeier H, Schoenauer R, Hostettler A, Ring A, Draeger A, Babiychuk EB (2014) Microvesicle shedding and lysosomal repair fulfill divergent cellular needs during the repair of streptolysin O-induced plasmalemmal damage. PLoS ONE 9:e89743 10.1371/journal.pone.0089743 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

