Unique immune characteristics and differential anti-PD-1-mediated reinvigoration potential of CD8+ TILs based on BRCA1/2 mutation status in epithelial ovarian cancers
- PMID: 38964784
- PMCID: PMC11227838
- DOI: 10.1136/jitc-2024-009058
Unique immune characteristics and differential anti-PD-1-mediated reinvigoration potential of CD8+ TILs based on BRCA1/2 mutation status in epithelial ovarian cancers
Abstract
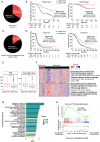
Background: We aimed to investigate the distinct immunological characteristics of the tumor immune microenvironment in epithelial ovarian cancer (EOC) according to BRCA1/2 mutations status and differential PD-1 expression levels.
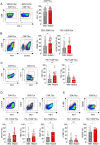
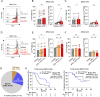
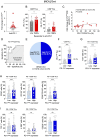
Methods: Tumor-infiltrating lymphocytes (TILs) were collected from patients with newly diagnosed advanced-stage EOC (YUHS cohort, n=117). This YUHS cohort was compared with The Cancer Genome Atlas (TCGA) data for ovarian serous cystadenocarcinoma (n=482), in terms of survival outcomes and immune-related gene profiles according to BRCA1/2 status. We used multicolor flow cytometry to characterize the immune phenotypes and heterogeneity of TILs with or without BRCA1/2 mutations. In vitro functional assays were conducted to evaluate the reinvigorating ability of CD8+ TILs on anti-PD-1 treatment.
Results: We found that EOC patients with BRCA1/2 mutations (BRCA1/2mt) exhibited better survival outcomes and significantly higher tumor mutation burden (TMB), compared with BRCA1/2 non-mutated (BRCA1/2wt) patients. Furthermore, CD8+ TILs within BRCA1/2mt tumors displayed characteristics indicating more severe T-cell exhaustion than their BRCA1/2wt counterparts. Notably, the capacity for anti-PD-1-mediated reinvigoration of CD8+ TILs was significantly greater in BRCA1/2wt tumors compared with BRCA1/2mt tumors. Additionally, within the BRCA1/2wt group, the frequency of PD-1highCD8+ TILs was positively correlated with the reinvigoration capacity of CD8+ TILs after anti-PD-1 treatment.
Conclusion: Our results highlight unique immune features of CD8+ TILs in EOC and a differential response to anti-PD-1 treatment, contingent on BRCA1/2 mutation status. These findings suggest that immune checkpoint blockade may be a promising frontline therapeutic option for selected BRCA1/2wt EOC patients.
Keywords: Immune Checkpoint Inhibitor; Ovarian Cancer; Tumor infiltrating lymphocyte - TIL.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: JYL reports grants and personal fees from AstraZeneca, Beigene, Bergenbio, Clovis Oncology, Immunogen, Janssen, Merck, MSD, Novartis, Roche, Seagen, Synthon, and Takeda. All other authors have no conflicts of interest to disclose.
Figures
Similar articles
-
Association and prognostic significance of BRCA1/2-mutation status with neoantigen load, number of tumor-infiltrating lymphocytes and expression of PD-1/PD-L1 in high grade serous ovarian cancer.Oncotarget. 2016 Mar 22;7(12):13587-98. doi: 10.18632/oncotarget.7277. Oncotarget. 2016. PMID: 26871470 Free PMC article.
-
4-1BB co-stimulation further enhances anti-PD-1-mediated reinvigoration of exhausted CD39+ CD8 T cells from primary and metastatic sites of epithelial ovarian cancers.J Immunother Cancer. 2020 Dec;8(2):e001650. doi: 10.1136/jitc-2020-001650. J Immunother Cancer. 2020. PMID: 33335029 Free PMC article.
-
Immune Checkpoint Inhibitor-induced Reinvigoration of Tumor-infiltrating CD8+ T Cells is Determined by Their Differentiation Status in Glioblastoma.Clin Cancer Res. 2019 Apr 15;25(8):2549-2559. doi: 10.1158/1078-0432.CCR-18-2564. Epub 2019 Jan 18. Clin Cancer Res. 2019. PMID: 30659023
-
The Impact of Neoadjuvant Chemotherapy on Ovarian Cancer Tumor Microenvironment: A Systematic Review of the Literature.Int J Mol Sci. 2024 Jun 27;25(13):7070. doi: 10.3390/ijms25137070. Int J Mol Sci. 2024. PMID: 39000178 Free PMC article. Review.
-
Cancer immunotherapy resistance based on immune checkpoints inhibitors: Targets, biomarkers, and remedies.Drug Resist Updat. 2020 Dec;53:100718. doi: 10.1016/j.drup.2020.100718. Epub 2020 Jul 15. Drug Resist Updat. 2020. PMID: 32736034 Review.
References
-
- Pujade-Lauraine E, Wagner U, Aavall-Lundqvist E, et al. . Pegylated liposomal doxorubicin and carboplatin compared with paclitaxel and carboplatin for patients with platinum-sensitive ovarian cancer in late relapse. Springer Healthcare; 2013. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
