Hsp47 promotes biogenesis of multi-subunit neuroreceptors in the endoplasmic reticulum
- PMID: 38963323
- PMCID: PMC11257679
- DOI: 10.7554/eLife.84798
Hsp47 promotes biogenesis of multi-subunit neuroreceptors in the endoplasmic reticulum
Abstract
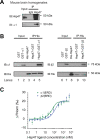
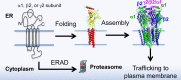
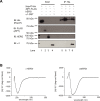
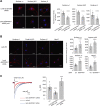
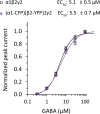
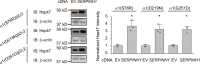
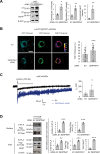
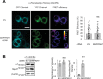
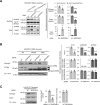
Protein homeostasis (proteostasis) deficiency is an important contributing factor to neurological and metabolic diseases. However, how the proteostasis network orchestrates the folding and assembly of multi-subunit membrane proteins is poorly understood. Previous proteomics studies identified Hsp47 (Gene: SERPINH1), a heat shock protein in the endoplasmic reticulum lumen, as the most enriched interacting chaperone for gamma-aminobutyric acid type A (GABAA) receptors. Here, we show that Hsp47 enhances the functional surface expression of GABAA receptors in rat neurons and human HEK293T cells. Furthermore, molecular mechanism study demonstrates that Hsp47 acts after BiP (Gene: HSPA5) and preferentially binds the folded conformation of GABAA receptors without inducing the unfolded protein response in HEK293T cells. Therefore, Hsp47 promotes the subunit-subunit interaction, the receptor assembly process, and the anterograde trafficking of GABAA receptors. Overexpressing Hsp47 is sufficient to correct the surface expression and function of epilepsy-associated GABAA receptor variants in HEK293T cells. Hsp47 also promotes the surface trafficking of other Cys-loop receptors, including nicotinic acetylcholine receptors and serotonin type 3 receptors in HEK293T cells. Therefore, in addition to its known function as a collagen chaperone, this work establishes that Hsp47 plays a critical and general role in the maturation of multi-subunit Cys-loop neuroreceptors.
Keywords: GABAA receptors; Hsp47; assembly; biochemistry; cell biology; chemical biology; epilepsy; folding; human; mouse; proteostasis.
© 2024, Wang, Di et al.
Conflict of interest statement
YW, XD, PZ, XC, MW, DH, RN, BH, FM, TM No competing interests declared
Figures





Update of
- doi: 10.1101/2022.10.24.513629
Similar articles
-
Remodeling the endoplasmic reticulum proteostasis network restores proteostasis of pathogenic GABAA receptors.PLoS One. 2018 Nov 27;13(11):e0207948. doi: 10.1371/journal.pone.0207948. eCollection 2018. PLoS One. 2018. PMID: 30481215 Free PMC article.
-
Combining valosin-containing protein (VCP) inhibition and suberanilohydroxamic acid (SAHA) treatment additively enhances the folding, trafficking, and function of epilepsy-associated γ-aminobutyric acid, type A (GABAA) receptors.J Biol Chem. 2015 Jan 2;290(1):325-37. doi: 10.1074/jbc.M114.580324. Epub 2014 Nov 18. J Biol Chem. 2015. PMID: 25406314 Free PMC article.
-
Quantitative interactome proteomics identifies a proteostasis network for GABAA receptors.J Biol Chem. 2022 Oct;298(10):102423. doi: 10.1016/j.jbc.2022.102423. Epub 2022 Aug 27. J Biol Chem. 2022. PMID: 36030824 Free PMC article.
-
HSP47 as a collagen-specific molecular chaperone: function and expression in normal mouse development.Semin Cell Dev Biol. 2003 Oct;14(5):275-82. doi: 10.1016/j.semcdb.2003.09.020. Semin Cell Dev Biol. 2003. PMID: 14986857 Review.
-
Roles of the endoplasmic reticulum-resident, collagen-specific molecular chaperone Hsp47 in vertebrate cells and human disease.J Biol Chem. 2019 Feb 8;294(6):2133-2141. doi: 10.1074/jbc.TM118.002812. Epub 2018 Dec 12. J Biol Chem. 2019. PMID: 30541925 Free PMC article. Review.
Cited by
-
HSP47 in human diseases: Navigating pathophysiology, diagnosis and therapy.Clin Transl Med. 2024 Aug;14(8):e1755. doi: 10.1002/ctm2.1755. Clin Transl Med. 2024. PMID: 39135385 Free PMC article. Review.
-
Pharmacological chaperones restore proteostasis of epilepsy-associated GABAA receptor variants.bioRxiv [Preprint]. 2023 Apr 19:2023.04.18.537383. doi: 10.1101/2023.04.18.537383. bioRxiv. 2023. Update in: Pharmacol Res. 2024 Oct;208:107356. doi: 10.1016/j.phrs.2024.107356. PMID: 37131660 Free PMC article. Updated. Preprint.
-
Pharmacological chaperones restore proteostasis of epilepsy-associated GABAA receptor variants.Pharmacol Res. 2024 Oct;208:107356. doi: 10.1016/j.phrs.2024.107356. Epub 2024 Aug 30. Pharmacol Res. 2024. PMID: 39216838 Free PMC article.
References
-
- Bianchi FT, Camera P, Ala U, Imperiale D, Migheli A, Boda E, Tempia F, Berto G, Bosio Y, Oddo S, LaFerla FM, Taraglio S, Dotti CG, Di Cunto F. The collagen chaperone HSP47 is a new interactor of APP that affects the levels of extracellular beta-amyloid peptides. PLOS ONE. 2011;6:e22370. doi: 10.1371/journal.pone.0022370. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

