Therapeutic Implications and Regulations of Protein Post-translational Modifications in Parkinsons Disease
- PMID: 38960968
- PMCID: PMC11222187
- DOI: 10.1007/s10571-024-01471-8
Therapeutic Implications and Regulations of Protein Post-translational Modifications in Parkinsons Disease
Abstract
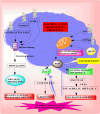
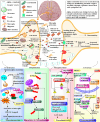
Parkinsons disease (PD) is a neurodegenerative disorder characterized by dopaminergic neuron loss and alpha-synuclein aggregation. This comprehensive review examines the intricate role of post-translational modifications (PTMs) in PD pathogenesis, focusing on DNA methylation, histone modifications, phosphorylation, SUMOylation, and ubiquitination. Targeted PTM modulation, particularly in key proteins like Parkin, DJ1, and PINK1, emerges as a promising therapeutic strategy for mitigating dopaminergic degeneration in PD. Dysregulated PTMs significantly contribute to the accumulation of toxic protein aggregates and dopaminergic neuronal dysfunction observed in PD. Targeting PTMs, including epigenetic strategies, addressing aberrant phosphorylation events, and modulating SUMOylation processes, provides potential avenues for intervention. The ubiquitin-proteasome system, governed by enzymes like Parkin and Nedd4, offers potential targets for clearing misfolded proteins and developing disease-modifying interventions. Compounds like ginkgolic acid, SUMO E1 enzyme inhibitors, and natural compounds like Indole-3-carbinol illustrate the feasibility of modulating PTMs for therapeutic purposes in PD. This review underscores the therapeutic potential of PTM-targeted interventions in modulating PD-related pathways, emphasizing the need for further research in this promising area of Parkinsons disease therapeutics.
Keywords: Dopaminergic degeneration; PTM-targeted interventions; Parkinsons disease; Post-translational modifications; Protein aggregates.
© 2024. The Author(s).
Conflict of interest statement
There are no conflicts of interest.
Figures
Similar articles
-
Post-translational modifications of Parkinson's disease-related proteins: Phosphorylation, SUMOylation and Ubiquitination.Biochim Biophys Acta Mol Basis Dis. 2019 Aug 1;1865(8):2001-2007. doi: 10.1016/j.bbadis.2018.10.025. Epub 2018 Nov 6. Biochim Biophys Acta Mol Basis Dis. 2019. PMID: 30412791 Review.
-
The role of posttranslational modifications of α-synuclein and LRRK2 in Parkinson's disease: Potential contributions of environmental factors.Biochim Biophys Acta Mol Basis Dis. 2019 Aug 1;1865(8):1992-2000. doi: 10.1016/j.bbadis.2018.11.017. Epub 2018 Nov 24. Biochim Biophys Acta Mol Basis Dis. 2019. PMID: 30481588 Free PMC article. Review.
-
Targeting α-synuclein post-translational modifications in Parkinson's disease.Behav Brain Res. 2023 Feb 15;439:114204. doi: 10.1016/j.bbr.2022.114204. Epub 2022 Nov 11. Behav Brain Res. 2023. PMID: 36372243 Review.
-
Current Technologies Unraveling the Significance of Post-Translational Modifications (PTMs) as Crucial Players in Neurodegeneration.Biomolecules. 2024 Jan 16;14(1):118. doi: 10.3390/biom14010118. Biomolecules. 2024. PMID: 38254718 Free PMC article. Review.
-
Post-translational modifications: Regulators of neurodegenerative proteinopathies.Ageing Res Rev. 2021 Jul;68:101336. doi: 10.1016/j.arr.2021.101336. Epub 2021 Mar 26. Ageing Res Rev. 2021. PMID: 33775891 Review.
References
-
- Altay MF, Kumar ST, Burtscher J, Jagannath S, Strand C, Miki Y, Parkkinen L, Holton JL, Lashuel HA. Development and validation of an expanded antibody toolset that captures alpha-synuclein pathological diversity in Lewy body diseases. NPJ Parkinsons Dis. 2023;9:161. doi: 10.1038/s41531-023-00604-y. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

