Molecular pathway of anticancer effect of next-generation HSP90 inhibitors XL-888 and Debio0932 in neuroblastoma cell line
- PMID: 38958814
- PMCID: PMC11222184
- DOI: 10.1007/s12032-024-02428-z
Molecular pathway of anticancer effect of next-generation HSP90 inhibitors XL-888 and Debio0932 in neuroblastoma cell line
Abstract
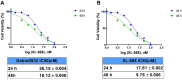
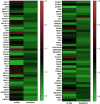
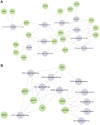
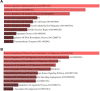
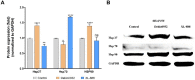
Neuroblastoma is a common nervous system tumor in childhood, and current treatments are not adequate. HSP90 is a molecular chaperone protein that plays a critical role in the regulation of cancer-related proteins. HSP90 inhibition may exert anticancer effects by targeting cancer-related processes such as tumor growth, cell proliferation, metastasis, and apoptosis. Therefore, HSP90 inhibition is a promising strategy in the treatment of various types of cancer, and the development of next-generation inhibitors could potentially lead to more effective and safer treatments. XL-888 and Debio0932 is a next-generation HSP90 inhibitor and can inhibit the correct folding and stabilization of client proteins that cancer-associated HSP90 helps to fold correctly. In this study, we aimed to investigate the comprehensive molecular pathways of the anticancer activity of XL-888 and Debio0932 in human neuroblastoma cells SH-SY5Y. The cytotoxic effects of XL-888 and Debio0932 on the neuroblastoma cell line SH-SY5Y cells were evaluated by MTT assay. Then, the effect of these HSP90 inhibitors on the expression of important genes in cancer was revealed by Quantitative Real Time Polymerase Chain Reaction (qRT-PCR) method. The qRT-PCR data were evaluated using Kyoto Encyclopedia of Genes and Genomes (KEGG) and Gene Ontology (GO) biological process tools. Finally, the effect of HSP90 inhibitors on HSP27, HSP70 and HSP90 protein expression was investigated by Western blotting analysis. The results revealed that XL-888 and Debio0932 had a role in regulating many cancer-related pathways such as migration, invasion, metastasis, angiogenesis, and apoptosis in SH-SY5Y cells. In conclusion, it shows that HSP90 inhibitors can be considered as a promising candidate in the treatment of neuroblastoma and resistance to chemotherapy.
Keywords: Cancer; Debio0932; HSP90 inhibition; Neuroblastoma; XL-888.
© 2024. The Author(s).
Conflict of interest statement
The authors confirm that this article content has no conflicts of interest.
Figures
Similar articles
-
Complex crystal structure determination and anti-non-small-cell lung cancer activity of the Hsp90N inhibitor Debio0932.Acta Crystallogr D Struct Biol. 2021 Jan 1;77(Pt 1):86-97. doi: 10.1107/S2059798320014990. Epub 2021 Jan 1. Acta Crystallogr D Struct Biol. 2021. PMID: 33404528
-
Investigation of the anti-cancer potential of epoxyazadiradione in neuroblastoma: experimental assays and molecular analysis.J Biomol Struct Dyn. 2024;42(21):11377-11395. doi: 10.1080/07391102.2023.2262593. Epub 2023 Sep 27. J Biomol Struct Dyn. 2024. PMID: 37753734
-
Suppression of heat shock protein 27 using OGX-427 induces endoplasmic reticulum stress and potentiates heat shock protein 90 inhibitors to delay castrate-resistant prostate cancer.Eur Urol. 2014 Jul;66(1):145-55. doi: 10.1016/j.eururo.2013.12.019. Epub 2013 Dec 29. Eur Urol. 2014. PMID: 24411988 Free PMC article.
-
HSP90 as a new therapeutic target for cancer therapy: the story unfolds.Expert Opin Biol Ther. 2002 Jan;2(1):3-24. doi: 10.1517/14712598.2.1.3. Expert Opin Biol Ther. 2002. PMID: 11772336 Review.
-
Recent advances in Hsp90 inhibitors as antitumor agents.Anticancer Agents Med Chem. 2008 Oct;8(7):761-82. doi: 10.2174/187152008785914824. Anticancer Agents Med Chem. 2008. PMID: 18855578 Review.
References
-
- Magwenyane AM, Ugbaja SC, Amoako DG, Somboro AM, Khan RB, Kumalo HM. Heat shock protein 90 (HSP90) inhibitors as anticancer medicines: a review on the computer-aided drug discovery approaches over the past 5 years. Comput Math Methods Med. 2022;2022:2147763. 10.1155/2022/2147763. 10.1155/2022/2147763 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

