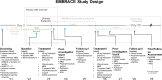
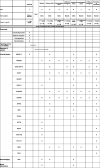
Engaging Mood Brain Circuits with Psilocybin (EMBRACE): a study protocol for a randomized, placebo-controlled and delayed-start, neuroimaging trial in depression
- PMID: 38956594
- PMCID: PMC11221029
- DOI: 10.1186/s13063-024-08268-6
Engaging Mood Brain Circuits with Psilocybin (EMBRACE): a study protocol for a randomized, placebo-controlled and delayed-start, neuroimaging trial in depression
Abstract
Background: Major depressive disorder (MDD) is a leading cause of disability worldwide across domains of health and cognition, affecting overall quality of life. Approximately one third of individuals with depression do not fully respond to treatments (e.g., conventional antidepressants, psychotherapy) and alternative strategies are needed. Recent early phase trials suggest psilocybin may be a safe and efficacious intervention with rapid-acting antidepressant properties. Psilocybin is thought to exert therapeutic benefits by altering brain network connectivity and inducing neuroplastic changes that endure for weeks post-treatment. Although early clinical results are encouraging, psilocybin's acute neurobiological effects on neuroplasticity have not been fully investigated. We aim to examine for the first time how psilocybin acutely (intraday) and subacutely (weeks) alters functional brain networks implicated in depression.
Methods: Fifty participants diagnosed with MDD or persistent depressive disorder (PDD) will be recruited from a tertiary mood disorders clinic and undergo 1:1 randomization into either an experimental or control arm. Participants will be given either 25 mg psilocybin or 25 mg microcrystalline cellulose (MCC) placebo for the first treatment. Three weeks later, those in the control arm will transition to receiving 25 mg psilocybin. We will investigate whether treatments are associated with changes in arterial spin labelling and blood oxygenation level-dependent contrast neuroimaging assessments at acute and subacute timepoints. Primary outcomes include testing whether psilocybin demonstrates acute changes in (1) cerebral blood flow and (2) functional brain activity in networks associated with mood regulation and depression when compared to placebo, along with changes in MADRS score over time compared to placebo. Secondary outcomes include changes across complementary clinical psychiatric, cognitive, and functional scales from baseline to final follow-up. Serum peripheral neurotrophic and inflammatory biomarkers will be collected at baseline and follow-up to examine relationships with clinical response, and neuroimaging measures.
Discussion: This study will investigate the acute and additive subacute neuroplastic effects of psilocybin on brain networks affected by depression using advanced serial neuroimaging methods. Results will improve our understanding of psilocybin's antidepressant mechanisms versus placebo response and whether biological measures of brain function can provide early predictors of treatment response.
Trial registration: ClinicalTrials.gov Identifier: NCT06072898. Registered on 6 October 2023.
Keywords: Arterial spin labelling; Cerebral blood flow; Depression; Functional magnetic resonance imaging; Major depressive disorder; Psilocybin; Psychedelics; Randomized controlled trial.
© 2024. The Author(s).
Conflict of interest statement
SMN has received startup salary and research support from the Sunnybrook Foundation and Department of Psychiatry. He has no financial conflicts of interest to declare.
Figures
Similar articles
-
Effects of Psilocybin-Assisted Therapy on Major Depressive Disorder: A Randomized Clinical Trial.JAMA Psychiatry. 2021 May 1;78(5):481-489. doi: 10.1001/jamapsychiatry.2020.3285. JAMA Psychiatry. 2021. PMID: 33146667 Free PMC article. Clinical Trial.
-
Sub-acute effects of psilocybin on EEG correlates of neural plasticity in major depression: Relationship to symptoms.J Psychopharmacol. 2023 Jul;37(7):687-697. doi: 10.1177/02698811231179800. Epub 2023 Jun 30. J Psychopharmacol. 2023. PMID: 37392016 Clinical Trial.
-
Increased global integration in the brain after psilocybin therapy for depression.Nat Med. 2022 Apr;28(4):844-851. doi: 10.1038/s41591-022-01744-z. Epub 2022 Apr 11. Nat Med. 2022. PMID: 35411074 Clinical Trial.
-
The Canadian Network for Mood and Anxiety Treatments (CANMAT) Task Force Report: Serotonergic Psychedelic Treatments for Major Depressive Disorder.Can J Psychiatry. 2023 Jan;68(1):5-21. doi: 10.1177/07067437221111371. Epub 2022 Aug 17. Can J Psychiatry. 2023. PMID: 35975555 Free PMC article. Review.
-
The role of psilocybin in depressive disorders.Curr Med Res Opin. 2024 Oct;40(10):1793-1808. doi: 10.1080/03007995.2024.2396536. Epub 2024 Aug 28. Curr Med Res Opin. 2024. PMID: 39177339 Review.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources