Boiling histotripsy exhibits anti-fibrotic effects in animal models of liver fibrosis
- PMID: 38956264
- PMCID: PMC11220065
- DOI: 10.1038/s41598-024-66078-x
Boiling histotripsy exhibits anti-fibrotic effects in animal models of liver fibrosis
Abstract
Liver fibrosis is a hallmark of chronic liver disease which could lead to liver cirrhosis or liver cancer. However, there is currently lack of a direct treatment for liver fibrosis. Boiling histotripsy (BH) is an emerging non-invasive high-intensity focused ultrasound technique that can be employed to mechanically destruct solid tumour at the focus via acoustic cavitation without significant adverse effect on surrounding tissue. Here, we investigated whether BH can mechanically fractionate liver fibrotic tissue thereby exhibiting an anti-fibrotic effect in an animal model of liver fibrosis. BH-treated penumbra and its identical lobe showed reduced liver fibrosis, accompanied by increased hepatocyte specific marker expression, compared to the BH-untreated lobe. Furthermore, BH treatment improved serological liver function markers without notable adverse effects. The ability of BH to reduce fibrosis and promote liver regeneration in liver fibrotic tissue suggests that BH could potentially be an effective and reliable therapeutic approach against liver fibrosis.
Keywords: Acoustic cavitation; Anti-fibrotic treatment; Boiling histotripsy; High intensity focused ultrasound; Liver fibrosis; Liver regeneration.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
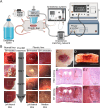
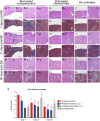
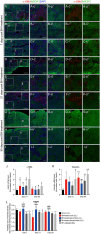
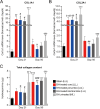

Similar articles
-
Investigation of the long-term healing response of the liver to boiling histotripsy treatment in vivo.Sci Rep. 2022 Aug 24;12(1):14462. doi: 10.1038/s41598-022-18544-7. Sci Rep. 2022. PMID: 36002564 Free PMC article.
-
Noninvasive mechanical destruction of liver tissue and tissue decellularisation by pressure-modulated shockwave histotripsy.Front Immunol. 2023 May 16;14:1150416. doi: 10.3389/fimmu.2023.1150416. eCollection 2023. Front Immunol. 2023. PMID: 37261363 Free PMC article.
-
Bubble dynamics in boiling histotripsy.Ultrasound Med Biol. 2018 Dec;44(12):2673-2696. doi: 10.1016/j.ultrasmedbio.2018.07.025. Epub 2018 Sep 15. Ultrasound Med Biol. 2018. PMID: 30228043
-
Histotripsy: A Method for Mechanical Tissue Ablation with Ultrasound.Annu Rev Biomed Eng. 2024 Jul;26(1):141-167. doi: 10.1146/annurev-bioeng-073123-022334. Epub 2024 Jun 20. Annu Rev Biomed Eng. 2024. PMID: 38346277 Free PMC article. Review.
-
Histotripsy: an innovative approach for minimally invasive tumour and disease treatment.Ann Med Surg (Lond). 2024 Mar 5;86(4):2081-2087. doi: 10.1097/MS9.0000000000001897. eCollection 2024 Apr. Ann Med Surg (Lond). 2024. PMID: 38576932 Free PMC article. Review.
References
-
- GBD 2015 Risk Factors Collaborators Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1659–1724. doi: 10.1016/S0140-6736(16)31679-8. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

