Targeting delivery of miR-146a via IMTP modified milk exosomes exerted cardioprotective effects by inhibiting NF-κB signaling pathway after myocardial ischemia-reperfusion injury
- PMID: 38951872
- PMCID: PMC11218161
- DOI: 10.1186/s12951-024-02631-0
Targeting delivery of miR-146a via IMTP modified milk exosomes exerted cardioprotective effects by inhibiting NF-κB signaling pathway after myocardial ischemia-reperfusion injury
Abstract
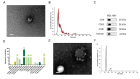
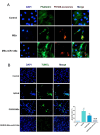
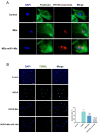
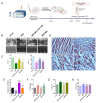
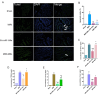
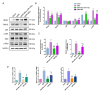
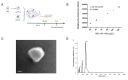
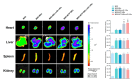
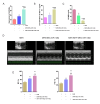
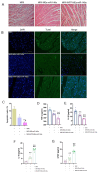
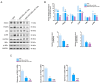
Reperfusion therapy is critical for saving heart muscle after myocardial infarction, but the process of restoring blood flow can itself exacerbate injury to the myocardium. This phenomenon is known as myocardial ischemia-reperfusion injury (MIRI), which includes oxidative stress, inflammation, and further cell death. microRNA-146a (miR-146a) is known to play a significant role in regulating the immune response and inflammation, and has been studied for its potential impact on the improvement of heart function after myocardial injury. However, the delivery of miR-146a to the heart in a specific and efficient manner remains a challenge as extracellular RNAs are unstable and rapidly degraded. Milk exosomes (MEs) have been proposed as ideal delivery platform for miRNA-based therapy as they can protect miRNAs from RNase degradation. In this study, the effects of miR-146a containing MEs (MEs-miR-146a) on improvement of cardiac function were examined in a rat model of MIRI. To enhance the targeting delivery of MEs-miR-146a to the site of myocardial injury, the ischemic myocardium-targeted peptide IMTP was modified onto the surfaces, and whether the modified MEs-miR-146a could exert a better therapeutic role was examined by echocardiography, myocardial injury indicators and the levels of inflammatory factors. Furthermore, the expressions of miR-146a mediated NF-κB signaling pathway-related proteins were detected by western blotting and qRT-PCR to further elucidate its mechanisms. MiR-146 mimics were successfully loaded into the MEs by electroporation at a square wave 1000 V voltage and 0.1 ms pulse duration. MEs-miR-146a can be up-taken by cardiomyocytes and protected the cells from oxygen glucose deprivation/reperfusion induced damage in vitro. Oral administration of MEs-miR-146a decreased myocardial tissue apoptosis and the expression of inflammatory factors and improved cardiac function after MIRI. The miR-146a level in myocardium tissues was significantly increased after the administration IMTP modified MEs-miR-146a, which was higher than that of the MEs-miR-146a group. In addition, intravenous injection of IMTP modified MEs-miR-146a enhanced the targeting to heart, improved cardiac function, reduced myocardial tissue apoptosis and suppressed inflammation after MIRI, which was more effective than the MEs-miR-146a treatment. Moreover, IMTP modified MEs-miR-146a reduced the protein levels of IRAK1, TRAF6 and p-p65. Therefore, IMTP modified MEs-miR-146a exerted their anti-inflammatory effect by inhibiting the IRAK1/TRAF6/NF-κB signaling pathway. Taken together, our findings suggested miR-146a containing MEs may be a promising strategy for the treatment of MIRI with better outcome after modification with ischemic myocardium-targeted peptide, which was expected to be applied in clinical practice in future.
Keywords: Inflammatory factors; Milk exosome; Myocardial ischemia-reperfusion injury; NF-κB signaling pathway; Targeting delivery; microRNA-146a.
© 2024. The Author(s).
Conflict of interest statement
TThe authors declare that they have no conflicts of interest.
Figures
Similar articles
-
Dexmedetomidine exerts cardioprotective effect through miR-146a-3p targeting IRAK1 and TRAF6 via inhibition of the NF-κB pathway.Biomed Pharmacother. 2021 Jan;133:110993. doi: 10.1016/j.biopha.2020.110993. Epub 2020 Nov 18. Biomed Pharmacother. 2021. PMID: 33220608
-
Human urine-derived stem cells protect against renal ischemia/reperfusion injury in a rat model via exosomal miR-146a-5p which targets IRAK1.Theranostics. 2020 Jul 25;10(21):9561-9578. doi: 10.7150/thno.42153. eCollection 2020. Theranostics. 2020. PMID: 32863945 Free PMC article.
-
Exosomes of bone-marrow stromal cells inhibit cardiomyocyte apoptosis under ischemic and hypoxic conditions via miR-486-5p targeting the PTEN/PI3K/AKT signaling pathway.Thromb Res. 2019 May;177:23-32. doi: 10.1016/j.thromres.2019.02.002. Epub 2019 Feb 2. Thromb Res. 2019. PMID: 30844685
-
The Role of NF-κB in Myocardial Ischemia/Reperfusion Injury.Curr Protein Pept Sci. 2022;23(8):535-547. doi: 10.2174/1389203723666220817085941. Curr Protein Pept Sci. 2022. PMID: 35980051 Review.
-
MicroRNA-specific therapeutic targets and biomarkers of apoptosis following myocardial ischemia-reperfusion injury.Mol Cell Biochem. 2024 Oct;479(10):2499-2521. doi: 10.1007/s11010-023-04876-z. Epub 2023 Oct 25. Mol Cell Biochem. 2024. PMID: 37878166 Review.
Cited by
-
Unveiling the therapeutic potential of miR-146a: Targeting innate inflammation in atherosclerosis.J Cell Mol Med. 2024 Oct;28(19):e70121. doi: 10.1111/jcmm.70121. J Cell Mol Med. 2024. PMID: 39392102 Free PMC article. Review.
-
Targeted delivery of extracellular vesicles: the mechanisms, techniques and therapeutic applications.Mol Biomed. 2024 Nov 21;5(1):60. doi: 10.1186/s43556-024-00230-x. Mol Biomed. 2024. PMID: 39567444 Free PMC article. Review.
-
Nanomedicines as Guardians of the Heart: Unleashing the Power of Antioxidants to Alleviate Myocardial Ischemic Injury.Theranostics. 2024 Aug 26;14(13):5336-5370. doi: 10.7150/thno.99961. eCollection 2024. Theranostics. 2024. PMID: 39267789 Free PMC article. Review.
-
Divergent Processing of Cell Stress Signals as the Basis of Cancer Progression: Licensing NFκB on Chromatin.Int J Mol Sci. 2024 Aug 7;25(16):8621. doi: 10.3390/ijms25168621. Int J Mol Sci. 2024. PMID: 39201306 Free PMC article. Review.
References
-
- McCartney PJ, Eteiba H, Maznyczka AM, McEntegart M, Greenwood JP, Muir DF, Chowdhary S, Gershlick AH, Appleby C, Cotton JM, Wragg A, Curzen N, Oldroyd KG, Lindsay M, Rocchiccioli JP, Shaukat A, Good R, Watkins S, Robertson K, Malkin C, Martin L, Gillespie L, Ford TJ, Petrie MC, Macfarlane PW, Tait RC, Welsh P, Sattar N, Weir RA, Fox KA, Ford I, McConnachie A, Berry C, T-TIME Group Effect of low-dose Intracoronary Alteplase during primary percutaneous coronary intervention on microvascular obstruction in patients with Acute myocardial infarction: a Randomized Clinical Trial. JAMA. 2019;321(1):56–68. doi: 10.1001/jama.2018.19802. - DOI - PMC - PubMed
-
- Heusch G, Gersh BJ. The pathophysiology of acute myocardial infarction and strategies of protection beyond reperfusion: a continual challenge. Eur Heart J. 2017;38(11):774–84. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

