Utilizing human cerebral organoids to model breast cancer brain metastasis in culture
- PMID: 38951862
- PMCID: PMC11218086
- DOI: 10.1186/s13058-024-01865-y
Utilizing human cerebral organoids to model breast cancer brain metastasis in culture
Abstract
Background: Metastasis, the spread, and growth of malignant cells at secondary sites within a patient's body, accounts for over 90% of cancer-related mortality. Breast cancer is the most common tumor type diagnosed and the leading cause of cancer lethality in women in the United States. It is estimated that 10-16% breast cancer patients will have brain metastasis. Current therapies to treat patients with breast cancer brain metastasis (BCBM) remain palliative. This is largely due to our limited understanding of the fundamental molecular and cellular mechanisms through which BCBM progresses, which represents a critical barrier for the development of efficient therapies for affected breast cancer patients.
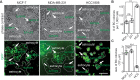
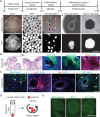
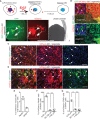
Methods: Previous research in BCBM relied on co-culture assays of tumor cells with rodent neural cells or rodent brain slice ex vivo. Given the need to overcome the obstacle for human-relevant host to study cell-cell communication in BCBM, we generated human embryonic stem cell-derived cerebral organoids to co-culture with human breast cancer cell lines. We used MDA-MB-231 and its brain metastatic derivate MDA-MB-231 Br-EGFP, other cell lines of MCF-7, HCC-1806, and SUM159PT. We leveraged this novel 3D co-culture platform to investigate the crosstalk of human breast cancer cells with neural cells in cerebral organoid.
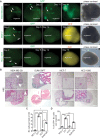
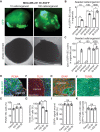
Results: We found that MDA-MB-231 and SUM159PT breast cancer cells formed tumor colonies in human cerebral organoids. Moreover, MDA-MB-231 Br-EGFP cells showed increased capacity to invade and expand in human cerebral organoids.
Conclusions: Our co-culture model has demonstrated a remarkable capacity to discern the brain metastatic ability of human breast cancer cells in cerebral organoids. The generation of BCBM-like structures in organoid will facilitate the study of human tumor microenvironment in culture.
Keywords: Brain metastasis; Breast cancer; Cell-cell communication; Cerebral organoids; Neural cells; Tumor microenvironment.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Impact of brain organoid-derived sEVs on metastatic adaptation and invasion of breast carcinoma cells through a microphysiological system.Lab Chip. 2024 Jul 10;24(14):3434-3455. doi: 10.1039/d4lc00296b. Lab Chip. 2024. PMID: 38888211
-
A simple metastatic brain cancer model using human embryonic stem cell-derived cerebral organoids.FASEB J. 2020 Dec;34(12):16464-16475. doi: 10.1096/fj.202000372R. Epub 2020 Oct 25. FASEB J. 2020. PMID: 33099835
-
Comprehensive analysis of differentially expressed long noncoding RNAs, miRNAs and mRNAs in breast cancer brain metastasis.Epigenomics. 2021 Jul;13(14):1113-1128. doi: 10.2217/epi-2021-0152. Epub 2021 Jun 21. Epigenomics. 2021. PMID: 34148372
-
Crosstalk between breast cancer-derived microRNAs and brain microenvironmental cells in breast cancer brain metastasis.Front Oncol. 2024 Aug 8;14:1436942. doi: 10.3389/fonc.2024.1436942. eCollection 2024. Front Oncol. 2024. PMID: 39175471 Free PMC article. Review.
-
Analysis of neuroglia and immune cells in the tumor microenvironment of breast cancer brain metastasis.Cancer Biol Ther. 2024 Dec 31;25(1):2398285. doi: 10.1080/15384047.2024.2398285. Epub 2024 Sep 5. Cancer Biol Ther. 2024. PMID: 39238191 Free PMC article. Review.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

