Advanced Glycation End Products-Induced Alzheimer's Disease and Its Novel Therapeutic Approaches: A Comprehensive Review
- PMID: 38947632
- PMCID: PMC11214645
- DOI: 10.7759/cureus.61373
Advanced Glycation End Products-Induced Alzheimer's Disease and Its Novel Therapeutic Approaches: A Comprehensive Review
Abstract
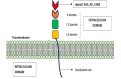
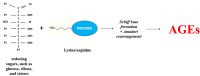
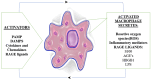
Advanced glycation end products (AGEs) accumulate in the brain, leading to neurodegenerative conditions such as Alzheimer's disease (AD). The pathophysiology of AD is influenced by receptors for AGEs and toll-like receptor 4 (TLR4). Protein glycation results in irreversible AGEs through a complicated series of reactions involving the formation of Schiff's base, the Amadori reaction, followed by the Maillard reaction, which causes abnormal brain glucose metabolism, oxidative stress, malfunctioning mitochondria, plaque deposition, and neuronal death. Amyloid plaque and other stimuli activate macrophages, which are crucial immune cells in AD development, triggering the production of inflammatory molecules and contributing to the disease's pathogenesis. The risk of AD is doubled by risk factors for atherosclerosis, dementia, advanced age, and type 2 diabetic mellitus (DM). As individuals age, the prevalence of neurological illnesses such as AD increases due to a decrease in glyoxalase levels and an increase in AGE accumulation. Insulin's role in proteostasis influences hallmarks of AD-like tau phosphorylation and amyloid β peptide clearance, affecting lipid metabolism, inflammation, vasoreactivity, and vascular function. The high-mobility group box 1 (HMGB1) protein, a key initiator and activator of a neuroinflammatory response, has been linked to the development of neurodegenerative diseases such as AD. The TLR4 inhibitor was found to improve memory and learning impairment and decrease Aβ build-up. Therapeutic research into anti-glycation agents, receptor for advanced glycation end products (RAGE) inhibitors, and AGE breakers offers hope for intervention strategies. Dietary and lifestyle modifications can also slow AD progression. Newer therapeutic approaches targeting AGE-related pathways are needed.
Keywords: ages; alzheimer’s disease; dementia; oxidative stress; rage.
Copyright © 2024, Kothandan et al.
Conflict of interest statement
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures
Similar articles
-
Impact of HMGB1, RAGE, and TLR4 in Alzheimer's Disease (AD): From Risk Factors to Therapeutic Targeting.Cells. 2020 Feb 7;9(2):383. doi: 10.3390/cells9020383. Cells. 2020. PMID: 32046119 Free PMC article. Review.
-
AGE-RAGE stress: a changing landscape in pathology and treatment of Alzheimer's disease.Mol Cell Biochem. 2019 Sep;459(1-2):95-112. doi: 10.1007/s11010-019-03553-4. Epub 2019 May 11. Mol Cell Biochem. 2019. PMID: 31079281 Review.
-
Receptor for Advanced Glycation End Products: Dementia and Cognitive Impairment.Drug Res (Stuttg). 2023 Jun;73(5):247-250. doi: 10.1055/a-2015-8041. Epub 2023 Mar 8. Drug Res (Stuttg). 2023. PMID: 36889338 Review.
-
Decoding Alzheimer's disease from perturbed cerebral glucose metabolism: implications for diagnostic and therapeutic strategies.Prog Neurobiol. 2013 Sep;108:21-43. doi: 10.1016/j.pneurobio.2013.06.004. Epub 2013 Jul 11. Prog Neurobiol. 2013. PMID: 23850509 Review.
-
HMGB1/RAGE/TLR4 axis and glutamate as novel targets for PCSK9 inhibitor in high fat cholesterol diet induced cognitive impairment and amyloidosis.Life Sci. 2021 May 15;273:119310. doi: 10.1016/j.lfs.2021.119310. Epub 2021 Mar 2. Life Sci. 2021. PMID: 33667517
References
-
- Why women have more Alzheimer's disease than men: gender and mitochondrial toxicity of amyloid-beta peptide. Viña J, Lloret A. J Alzheimers Dis. 2010;20 Suppl 2:0–33. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
