Steering the course of CAR T cell therapy with lipid nanoparticles
- PMID: 38943167
- PMCID: PMC11212433
- DOI: 10.1186/s12951-024-02630-1
Steering the course of CAR T cell therapy with lipid nanoparticles
Abstract
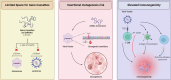
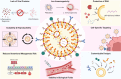
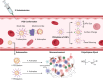
Lipid nanoparticles (LNPs) have proven themselves as transformative actors in chimeric antigen receptor (CAR) T cell therapy, surpassing traditional methods and addressing challenges like immunogenicity, reduced toxicity, and improved safety. Promising preclinical results signal a shift toward safer and more effective CAR T cell treatments. Ongoing research aims to validate these findings in clinical trials, marking a new era guided by LNPs utility in CAR therapy. Herein, we explore the preference for LNPs over traditional methods, highlighting the versatility of LNPs and their effective delivery of nucleic acids. Additionally, we address key challenges in clinical considerations, heralding a new era in CAR T cell therapy.
Keywords: Chimeric antigen receptor; Immunotherapy; Lipid nanoparticles; Nonviral transduction; mRNA delivery.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no potential conflicts of interest.
Figures


Similar articles
-
Synergistic integration of mRNA-LNP with CAR-engineered immune cells: Pioneering progress in immunotherapy.Mol Ther. 2024 Nov 6;32(11):3772-3792. doi: 10.1016/j.ymthe.2024.09.019. Epub 2024 Sep 17. Mol Ther. 2024. PMID: 39295145 Review.
-
Antigen Presenting Cell Mimetic Lipid Nanoparticles for Rapid mRNA CAR T Cell Cancer Immunotherapy.Adv Mater. 2024 Jun;36(26):e2313226. doi: 10.1002/adma.202313226. Epub 2024 Mar 15. Adv Mater. 2024. PMID: 38419362
-
Lipid nanoparticle (LNP) mediated mRNA delivery in cardiovascular diseases: Advances in genome editing and CAR T cell therapy.J Control Release. 2024 Aug;372:113-140. doi: 10.1016/j.jconrel.2024.06.023. Epub 2024 Jun 15. J Control Release. 2024. PMID: 38876358 Review.
-
Nanotechnology and immunoengineering: How nanotechnology can boost CAR-T therapy.Acta Biomater. 2020 Jun;109:21-36. doi: 10.1016/j.actbio.2020.04.015. Epub 2020 Apr 13. Acta Biomater. 2020. PMID: 32294554 Review.
-
Immune Cell Hacking: Challenges and Clinical Approaches to Create Smarter Generations of Chimeric Antigen Receptor T Cells.Front Immunol. 2018 Jul 31;9:1717. doi: 10.3389/fimmu.2018.01717. eCollection 2018. Front Immunol. 2018. PMID: 30108584 Free PMC article. Review.
References
-
- Stanley JO, Mohamed SA. Cancer immunotherapy: a brief review of the history, possibilities, and challenges ahead. J Cancer Metastasis Treat. 2017;3:250–261. doi: 10.20517/2394-4722.2017.41. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

