Advancing drug development for atrial fibrillation by prioritising findings from human genetic association studies
- PMID: 38941956
- PMCID: PMC11260865
- DOI: 10.1016/j.ebiom.2024.105194
Advancing drug development for atrial fibrillation by prioritising findings from human genetic association studies
Abstract
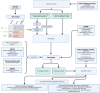
Background: Drug development for atrial fibrillation (AF) has failed to yield new approved compounds. We sought to identify and prioritise potential druggable targets with support from human genetics, by integrating the available evidence with bioinformatics sources relevant for AF drug development.
Methods: Genetic hits for AF and related traits were identified through structured search of MEDLINE. Genes derived from each paper were cross-referenced with the OpenTargets platform for drug interactions. Confirmation/validation was demonstrated through structured searches and review of evidence on MEDLINE and ClinialTrials.gov for each drug and its association with AF.
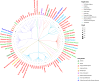
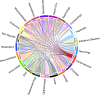
Findings: 613 unique drugs were identified, with 21 already included in AF Guidelines. Cardiovascular drugs from classes not currently used for AF (e.g. ranolazine and carperitide) and anti-inflammatory drugs (e.g. dexamethasone and mehylprednisolone) had evidence of potential benefit. Further targets were considered druggable but remain open for drug development.
Interpretation: Our systematic approach, combining evidence from different bioinformatics platforms, identified drug repurposing opportunities and druggable targets for AF.
Funding: KK is supported by Barts Charity grant G-002089 and is mentored on the AFGen 2023-24 Fellowship funded by the AFGen NIH/NHLBI grant R01HL092577. RP is supported by the UCL BHF Research Accelerator AA/18/6/34223 and NIHR grant NIHR129463. AFS is supported by the BHF grants PG/18/5033837, PG/22/10989 and UCL BHF Accelerator AA/18/6/34223 as well as the UK Research and Innovation (UKRI) under the UK government's Horizon Europe funding guarantee EP/Z000211/1 and by the UKRI-NIHR grant MR/V033867/1 for the Multimorbidity Mechanism and Therapeutics Research Collaboration. AF is supported by UCL BHF Accelerator AA/18/6/34223. CF is supported by UCL BHF Accelerator AA/18/6/34223.
Keywords: Atrial fibrillation; Bioinformatics; Drug development; GWAS; Systematic review.
Copyright © 2024 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests AFS and CF have received unrestricted funding from New Amsterdam Pharma, for investigating the role of a specific drug not related to AF. CF has received funding from Pfizer investigating the role of specific drug targets not related to AF, he is not the principal investigator on this. PL has received grants from Boston Scientific and Abbott Medical as well as consulting fees from Boston Scientific. RJS has received grants, consulting fees and honoraria from Abbott medical, Biosense Webster, Boston Scientific and Medtronic.
Figures

Similar articles
-
Curative catheter ablation in atrial fibrillation and typical atrial flutter: systematic review and economic evaluation.Health Technol Assess. 2008 Nov;12(34):iii-iv, xi-xiii, 1-198. doi: 10.3310/hta12340. Health Technol Assess. 2008. PMID: 19036232 Review.
-
Ranolazine for the treatment of atrial fibrillation.Expert Opin Investig Drugs. 2015 Jun;24(6):825-36. doi: 10.1517/13543784.2015.1036984. Epub 2015 Apr 15. Expert Opin Investig Drugs. 2015. PMID: 25872749 Review.
-
A New Therapeutic Framework for Atrial Fibrillation Drug Development.Circ Res. 2020 Jun 19;127(1):184-201. doi: 10.1161/CIRCRESAHA.120.316576. Epub 2020 Jun 18. Circ Res. 2020. PMID: 32717173 Review.
-
Mechanisms and Drug Development in Atrial Fibrillation.Pharmacol Rev. 2018 Jul;70(3):505-525. doi: 10.1124/pr.117.014183. Pharmacol Rev. 2018. PMID: 29921647 Free PMC article. Review.
-
Emerging Antiarrhythmic Drugs for Atrial Fibrillation.Int J Mol Sci. 2022 Apr 7;23(8):4096. doi: 10.3390/ijms23084096. Int J Mol Sci. 2022. PMID: 35456912 Free PMC article. Review.
Cited by
-
Dexmedetomidine vs. propofol on arrhythmia in cardiac surgery: a meta-analysis of randomized controlled trials.Front Cardiovasc Med. 2024 Oct 10;11:1433841. doi: 10.3389/fcvm.2024.1433841. eCollection 2024. Front Cardiovasc Med. 2024. PMID: 39450236 Free PMC article.
-
Integrating metabolomics and proteomics to identify novel drug targets for heart failure and atrial fibrillation.Genome Med. 2024 Oct 21;16(1):120. doi: 10.1186/s13073-024-01395-4. Genome Med. 2024. PMID: 39434187 Free PMC article.
-
Lipid-lowering drugs, circulating inflammatory factors, and atrial fibrillation: a mediation Mendelian randomization study.Front Cardiovasc Med. 2024 Nov 5;11:1446610. doi: 10.3389/fcvm.2024.1446610. eCollection 2024. Front Cardiovasc Med. 2024. PMID: 39563941 Free PMC article.
References
-
- Gallagher C., Hendriks J.M., Giles L., et al. Increasing trends in hospitalisations due to atrial fibrillation in Australia from 1993 to 2013. Heart. 2019;105(17):1358–1363. - PubMed
-
- Ang Y.S., Rajamani S., Haldar S.M., Hüser J. A new therapeutic framework for atrial fibrillation drug development. Circ Res. 2020:184–201. - PubMed
-
- DNA sequencing costs: data. Genome.gov; 2022. https://www.genome.gov/about-genomics/fact-sheets/DNA-Sequencing-Costs-Data [cited 2023 May 10]. Available from:
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

