Poly (acrylic acid)/tricalcium phosphate nanoparticles scaffold enriched with exosomes for cell-free therapy in bone tissue engineering: An in vivo evaluation
- PMID: 38938758
- PMCID: PMC11199929
- DOI: 10.34172/bi.2023.27510
Poly (acrylic acid)/tricalcium phosphate nanoparticles scaffold enriched with exosomes for cell-free therapy in bone tissue engineering: An in vivo evaluation
Abstract
Introduction: This study aimed to assess the potential of poly (acrylic acid)/tricalcium phosphate nanoparticles (PAA/triCaPNPs) scaffold in terms of biocompatibility and osteoconductivity properties the in-vivo evaluation as well as to investigate the performance of PAA/triCaPNPs scaffold (with or without exosomes derived from UC-MSCs) for bone regeneration of rat critical-sized defect.
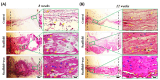
Methods: PAA/triCaPNPs scaffold was made from acrylic acid (AA) monomer, N,N'-methylenebisacrylamide (MBAA), sodium bicarbonate (SBC), and ammonium persulfate (APS) through freeze-drying method. For in vivo evaluation, we randomly divided 24 rats into three groups. The rat calvarial bone defects were treated as follows: (1) Control group: defects without any treatment, (2) scaffold group: defects treated with scaffold only, (3) scaffold+exo group: defects treated with scaffold enriched with exosomes (1 μg/μL, 150 μg per rat). Eight- and 12-weeks post-surgery, half of the animals were sacrificed and bone regeneration was examined through micro-computerized tomography (µ-CT), histological staining, and immunohistochemistry (IHC).
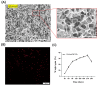
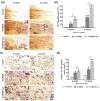
Results: Quantitative analysis based on µ-CT scan images at 8 and 12 weeks post-implantation clearly indicated that healing rate for defects that were filled with scaffold enriched with exosome was significantly higher than defects filled with scaffold without exosome. The H&E and Masson staining results revealed that more new bone-like form developed in the scaffold+exo group than that in control and scaffold groups. Further, IHC staining for osteocalcin and CD31 confirmed that more bone healing in the scaffold+exo group at 12 weeks could be associated with osteogenesis and angiogenesis concurrently.
Conclusion: In the present study, we aimed to investigate the therapeutic potential of PAA/triCaPNPs scaffold as a carrier of human UC-MSC-derived exosome to achieve the exosome-controlled release on calvarial bone defect. The in vivo results indicated that the exosome-enriched scaffold could effectively minify the defect area and improve the bone healing in rat model, and as such it could be an option for exosome-based therapy.
Keywords: Cell-free therapy; Critical-sized bone defect; PAA; Preclinical imaging; Tricalcium phosphate nanoparticles; UCMSC-exosome.
© 2024 The Author(s).
Conflict of interest statement
There is no competing financial interest.
Figures
Similar articles
-
Preparation of poly(acrylic acid)/tricalcium phosphate nanoparticles scaffold: Characterization and releasing UC-MSCs derived exosomes for bone differentiation.Bioimpacts. 2023;13(5):425-438. doi: 10.34172/bi.2022.24142. Epub 2022 Aug 22. Bioimpacts. 2023. PMID: 37736343 Free PMC article.
-
Effect of poly (lactide-co-glycolide) (PLGA)-coated beta-tricalcium phosphate on the healing of rat calvarial bone defects: a comparative study with pure-phase beta-tricalcium phosphate.Clin Oral Implants Res. 2016 Nov;27(11):1360-1367. doi: 10.1111/clr.12744. Epub 2016 Jan 8. Clin Oral Implants Res. 2016. PMID: 26748831
-
Evaluation of the effect of co-transplantation of collagen-hydroxyapatite bio-scaffold containing nanolycopene and human endometrial mesenchymal stem cell derived exosomes to regenerate bone in rat critical size calvarial defect.Regen Ther. 2024 Jul 2;26:387-400. doi: 10.1016/j.reth.2024.02.006. eCollection 2024 Jun. Regen Ther. 2024. PMID: 39045576 Free PMC article.
-
Engineering biomimetic periosteum with β-TCP scaffolds to promote bone formation in calvarial defects of rats.Stem Cell Res Ther. 2017 Jun 5;8(1):134. doi: 10.1186/s13287-017-0592-4. Stem Cell Res Ther. 2017. PMID: 28583167 Free PMC article.
-
Periosteum and development of the tissue-engineered periosteum for guided bone regeneration.J Orthop Translat. 2022 Feb 16;33:41-54. doi: 10.1016/j.jot.2022.01.002. eCollection 2022 Mar. J Orthop Translat. 2022. PMID: 35228996 Free PMC article. Review.
References
-
- Zhao Y, Li Z, Jiang Y, Liu H, Feng Y, Wang Z, et al. Bioinspired mineral hydrogels as nanocomposite scaffolds for the promotion of osteogenic marker expression and the induction of bone regeneration in osteoporosis. Acta Biomaterialia. 2020;113:614–26. doi: 10.1016/j.actbio.2020.06.024. - DOI - PubMed
-
- Wiltfang J, Zernial O, Behrens E, Schlegel A, Warnke PH, Becker ST. Regenerative treatment of peri-implantitis bone defects with a combination of autologous bone and a demineralized xenogenic bone graft: a series of 36 defects. Clin Implant Dent Relat Res. 2012;14:421–7. doi: 10.1111/j.1708-8208.2009.00264.x. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous