Unraveling the intricate relationship between lipid metabolism and oncogenic signaling pathways
- PMID: 38933330
- PMCID: PMC11199418
- DOI: 10.3389/fcell.2024.1399065
Unraveling the intricate relationship between lipid metabolism and oncogenic signaling pathways
Abstract
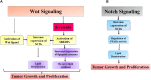
Lipids, the primary constituents of the cell membrane, play essential roles in nearly all cellular functions, such as cell-cell recognition, signaling transduction, and energy provision. Lipid metabolism is necessary for the maintenance of life since it regulates the balance between the processes of synthesis and breakdown. Increasing evidence suggests that cancer cells exhibit abnormal lipid metabolism, significantly affecting their malignant characteristics, including self-renewal, differentiation, invasion, metastasis, and drug sensitivity and resistance. Prominent oncogenic signaling pathways that modulate metabolic gene expression and elevate metabolic enzyme activity include phosphoinositide 3-kinase (PI3K)/AKT, MAPK, NF-kB, Wnt, Notch, and Hippo pathway. Conversely, when metabolic processes are not regulated, they can lead to malfunctions in cellular signal transduction pathways. This, in turn, enables uncontrolled cancer cell growth by providing the necessary energy, building blocks, and redox potentials. Therefore, targeting lipid metabolism-associated oncogenic signaling pathways could be an effective therapeutic approach to decrease cancer incidence and promote survival. This review sheds light on the interactions between lipid reprogramming and signaling pathways in cancer. Exploring lipid metabolism as a target could provide a promising approach for creating anticancer treatments by identifying metabolic inhibitors. Additionally, we have also provided an overview of the drugs targeting lipid metabolism in cancer in this review.
Keywords: anticancer therapy; cancer; cell signaling; lipid metabolism; oncogenic proteins.
Copyright © 2024 Khan, Elsori, Verma, Pandey, Obaidur Rab, Siddiqui, Alabdallah, Saeed and Pandey.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Cancer Metabolism: Phenotype, Signaling and Therapeutic Targets.Cells. 2020 Oct 16;9(10):2308. doi: 10.3390/cells9102308. Cells. 2020. PMID: 33081387 Free PMC article. Review.
-
Lipid metabolism alteration contributes to and maintains the properties of cancer stem cells.Theranostics. 2020 May 30;10(16):7053-7069. doi: 10.7150/thno.41388. eCollection 2020. Theranostics. 2020. PMID: 32641978 Free PMC article. Review.
-
NAD(P)H Quinone Dehydrogenase 1 Ablation Inhibits Activation of the Phosphoinositide 3-Kinase/Akt Serine/Threonine Kinase and Mitogen-Activated Protein Kinase/Extracellular Signal-Regulated Kinase Pathways and Blocks Metabolic Adaptation in Hepatocellular Carcinoma.Hepatology. 2020 Feb;71(2):549-568. doi: 10.1002/hep.30818. Epub 2019 Aug 19. Hepatology. 2020. PMID: 31215069 Free PMC article.
-
Targeting Lipid Metabolic Reprogramming as Anticancer Therapeutics.J Cancer Prev. 2016 Dec;21(4):209-215. doi: 10.15430/JCP.2016.21.4.209. Epub 2016 Dec 30. J Cancer Prev. 2016. PMID: 28053954 Free PMC article. Review.
-
Induction of metastasis, cancer stem cell phenotype, and oncogenic metabolism in cancer cells by ionizing radiation.Mol Cancer. 2017 Jan 30;16(1):10. doi: 10.1186/s12943-016-0577-4. Mol Cancer. 2017. PMID: 28137309 Free PMC article. Review.
References
-
- Airley R. E., McHugh P., Evans A. R., Harris B., Winchester L., Buffa F. M., et al. (2014). Role of carbohydrate response element-binding protein (ChREBP) in generating an aerobic metabolic phenotype and in breast cancer progression. Br. J. Cancer 110 (3), 715–723. 10.1038/bjc.2013.765 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

