Placental Epigenome Impacts Fetal Development: Effects of Maternal Nutrients and Gut Microbiota
- PMID: 38931215
- PMCID: PMC11206482
- DOI: 10.3390/nu16121860
Placental Epigenome Impacts Fetal Development: Effects of Maternal Nutrients and Gut Microbiota
Abstract
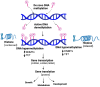
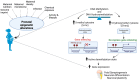
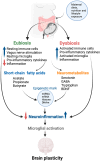
Evidence is emerging on the role of maternal diet, gut microbiota, and other lifestyle factors in establishing lifelong health and disease, which are determined by transgenerationally inherited epigenetic modifications. Understanding epigenetic mechanisms may help identify novel biomarkers for gestation-related exposure, burden, or disease risk. Such biomarkers are essential for developing tools for the early detection of risk factors and exposure levels. It is necessary to establish an exposure threshold due to nutrient deficiencies or other environmental factors that can result in clinically relevant epigenetic alterations that modulate disease risks in the fetus. This narrative review summarizes the latest updates on the roles of maternal nutrients (n-3 fatty acids, polyphenols, vitamins) and gut microbiota on the placental epigenome and its impacts on fetal brain development. This review unravels the potential roles of the functional epigenome for targeted intervention to ensure optimal fetal brain development and its performance in later life.
Keywords: brain development; fetal development; gut microbiota; immune function; maternal diet; placental epigenome.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
High-fat diet intake modulates maternal intestinal adaptations to pregnancy and results in placental hypoxia, as well as altered fetal gut barrier proteins and immune markers.J Physiol. 2019 Jun;597(12):3029-3051. doi: 10.1113/JP277353. Epub 2019 May 13. J Physiol. 2019. PMID: 31081119
-
A crucial role for maternal dietary methyl donor intake in epigenetic programming and fetal growth outcomes.Nutr Rev. 2018 Jun 1;76(6):469-478. doi: 10.1093/nutrit/nuy006. Nutr Rev. 2018. PMID: 29529267 Review.
-
From gut to placenta: understanding how the maternal microbiome models life-long conditions.Front Endocrinol (Lausanne). 2023 Dec 15;14:1304727. doi: 10.3389/fendo.2023.1304727. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 38161976 Free PMC article. Review.
-
Maternal Obesity and Gut Microbiota Are Associated with Fetal Brain Development.Nutrients. 2022 Oct 27;14(21):4515. doi: 10.3390/nu14214515. Nutrients. 2022. PMID: 36364776 Free PMC article. Review.
-
Maternal gut microbiota Bifidobacterium promotes placental morphogenesis, nutrient transport and fetal growth in mice.Cell Mol Life Sci. 2022 Jun 28;79(7):386. doi: 10.1007/s00018-022-04379-y. Cell Mol Life Sci. 2022. PMID: 35760917 Free PMC article.
References
-
- Duttaroy A.K. Docosahexaenoic acid supports feto-placental growth and protects cardiovascular and cognitive function: A mini review. Eur. J. Lipid Sci. Technol. 2016;118:1439–1449. doi: 10.1002/ejlt.201500496. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

