A Computational Model of Deep Brain Stimulation for Parkinson's Disease Tremor and Bradykinesia
- PMID: 38928620
- PMCID: PMC11201485
- DOI: 10.3390/brainsci14060620
A Computational Model of Deep Brain Stimulation for Parkinson's Disease Tremor and Bradykinesia
Abstract
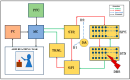
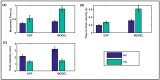
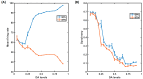
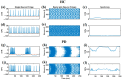
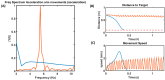
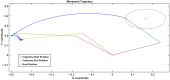
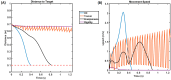
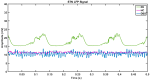
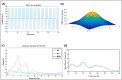
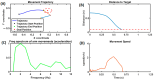
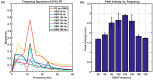
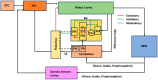
Parkinson's disease (PD) is a progressive neurological disorder that is typically characterized by a range of motor dysfunctions, and its impact extends beyond physical abnormalities into emotional well-being and cognitive symptoms. The loss of dopaminergic neurons in the substantia nigra pars compacta (SNc) leads to an array of dysfunctions in the functioning of the basal ganglia (BG) circuitry that manifests into PD. While active research is being carried out to find the root cause of SNc cell death, various therapeutic techniques are used to manage the symptoms of PD. The most common approach in managing the symptoms is replenishing the lost dopamine in the form of taking dopaminergic medications such as levodopa, despite its long-term complications. Another commonly used intervention for PD is deep brain stimulation (DBS). DBS is most commonly used when levodopa medication efficacy is reduced, and, in combination with levodopa medication, it helps reduce the required dosage of medication, prolonging the therapeutic effect. DBS is also a first choice option when motor complications such as dyskinesia emerge as a side effect of medication. Several studies have also reported that though DBS is found to be effective in suppressing severe motor symptoms such as tremors and rigidity, it has an adverse effect on cognitive capabilities. Henceforth, it is important to understand the exact mechanism of DBS in alleviating motor symptoms. A computational model of DBS stimulation for motor symptoms will offer great insights into understanding the mechanisms underlying DBS, and, along this line, in our current study, we modeled a cortico-basal ganglia circuitry of arm reaching, where we simulated healthy control (HC) and PD symptoms as well as the DBS effect on PD tremor and bradykinesia. Our modeling results reveal that PD tremors are more correlated with the theta band, while bradykinesia is more correlated with the beta band of the frequency spectrum of the local field potential (LFP) of the subthalamic nucleus (STN) neurons. With a DBS current of 220 pA, 130 Hz, and a 100 microsecond pulse-width, we could found the maximum therapeutic effect for the pathological dynamics simulated using our model using a set of parameter values. However, the exact DBS characteristics vary from patient to patient, and this can be further studied by exploring the model parameter space. This model can be extended to study different DBS targets and accommodate cognitive dynamics in the future to study the impact of DBS on cognitive symptoms and thereby optimize the parameters to produce optimal performance effects across modalities. Combining DBS with rehabilitation is another frontier where DBS can reduce symptoms such as tremors and rigidity, enabling patients to participate in their therapy. With DBS providing instant relief to patients, a combination of DBS and rehabilitation can enhance neural plasticity. One of the key motivations behind combining DBS with rehabilitation is to expect comparable results in motor performance even with milder DBS currents.
Keywords: Parkinson’s disease; basal ganglia; beta; bradykinesia; deep brain stimulation (DBS); dopamine; motor symptoms; rigidity; sub thalamic nucleus; theta; tremor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Subthalamic GAD gene transfer in Parkinson disease patients who are candidates for deep brain stimulation.Hum Gene Ther. 2001 Aug 10;12(12):1589-91. Hum Gene Ther. 2001. PMID: 11529246 Clinical Trial.
-
Beta-band oscillations in the supplementary motor cortex are modulated by levodopa and associated with functional activity in the basal ganglia.Neuroimage Clin. 2018 May 18;19:559-571. doi: 10.1016/j.nicl.2018.05.021. eCollection 2018. Neuroimage Clin. 2018. PMID: 29984164 Free PMC article.
-
Premotor cortical beta synchronization and the network neuromodulation of externally paced finger tapping in Parkinson's disease.Neurobiol Dis. 2024 Jul;197:106529. doi: 10.1016/j.nbd.2024.106529. Epub 2024 May 11. Neurobiol Dis. 2024. PMID: 38740349
-
An electrophysiological perspective on Parkinson's disease: symptomatic pathogenesis and therapeutic approaches.J Biomed Sci. 2021 Dec 9;28(1):85. doi: 10.1186/s12929-021-00781-z. J Biomed Sci. 2021. PMID: 34886870 Free PMC article. Review.
-
Deep brain stimulation for the treatment of Parkinson's disease.J Clin Neurophysiol. 2004 Jan-Feb;21(1):6-17. doi: 10.1097/00004691-200401000-00003. J Clin Neurophysiol. 2004. PMID: 15097290 Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

