A Narrative Review of the Dorsal Root Ganglia and Spinal Cord Mechanisms of Action of Neuromodulation Therapies in Neuropathic Pain
- PMID: 38928589
- PMCID: PMC11202229
- DOI: 10.3390/brainsci14060589
A Narrative Review of the Dorsal Root Ganglia and Spinal Cord Mechanisms of Action of Neuromodulation Therapies in Neuropathic Pain
Abstract
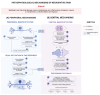
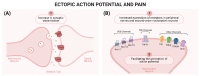
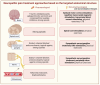
Neuropathic pain arises from injuries to the nervous system in diseases such as diabetes, infections, toxicity, and traumas. The underlying mechanism of neuropathic pain involves peripheral and central pathological modifications. Peripheral mechanisms entail nerve damage, leading to neuronal hypersensitivity and ectopic action potentials. Central sensitization involves a neuropathological process with increased responsiveness of the nociceptive neurons in the central nervous system (CNS) to their normal or subthreshold input due to persistent stimuli, leading to sustained electrical discharge, synaptic plasticity, and aberrant processing in the CNS. Current treatments, both pharmacological and non-pharmacological, aim to alleviate symptoms but often face challenges due to the complexity of neuropathic pain. Neuromodulation is emerging as an important therapeutic approach for the treatment of neuropathic pain in patients unresponsive to common therapies, by promoting the normalization of neuronal and/or glial activity and by targeting cerebral cortical regions, spinal cord, dorsal root ganglia, and nerve endings. Having a better understanding of the efficacy, adverse events and applicability of neuromodulation through pre-clinical studies is of great importance. Unveiling the mechanisms and characteristics of neuromodulation to manage neuropathic pain is essential to understand how to use it. In the present article, we review the current understanding supporting dorsal root ganglia and spinal cord neuromodulation as a therapeutic approach for neuropathic pain.
Keywords: central sensitization; current treatment; glial cells; neuromodulation; neuropathic pain.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Role of the immune system in neuropathic pain.Scand J Pain. 2019 Dec 18;20(1):33-37. doi: 10.1515/sjpain-2019-0138. Scand J Pain. 2019. PMID: 31730538 Review.
-
Elucidation of pathophysiology and treatment of neuropathic pain.Cent Nerv Syst Agents Med Chem. 2012 Dec;12(4):304-14. doi: 10.2174/187152412803760645. Cent Nerv Syst Agents Med Chem. 2012. PMID: 23033930 Review.
-
Pathobiology of neuropathic pain.Eur J Pharmacol. 2001 Oct 19;429(1-3):23-37. doi: 10.1016/s0014-2999(01)01303-6. Eur J Pharmacol. 2001. PMID: 11698024 Review.
-
Regional Hyperexcitability and Chronic Neuropathic Pain Following Spinal Cord Injury.Cell Mol Neurobiol. 2020 Aug;40(6):861-878. doi: 10.1007/s10571-020-00785-7. Epub 2020 Jan 18. Cell Mol Neurobiol. 2020. PMID: 31955281 Review.
-
Spinal Cord Stimulation.2023 Apr 24. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2023 Apr 24. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31985947 Free Books & Documents.
References
-
- Raja S.N., Carr D.B., Cohen M., Finnerup N.B., Flor H., Gibson S., Keefe F.J., Mogil J.S., Ringkamp M., Sluka K.A., et al. The Revised International Association for the Study of Pain Definition of Pain: Concepts, Challenges, and Compromises. Pain. 2020;161:1976–1982. doi: 10.1097/j.pain.0000000000001939. - DOI - PMC - PubMed
Publication types
Grants and funding
- #203112/2020-2, #307852/2019-9, #309633/2021-4, #405027/2021-4, and #427946/2018-2/National Council for Scientific and Technological Development
- PRONEX grant agreement #014/2017; PBA/PROPPG 13/2021 agreements #276/2022-PBA and #250/2022-PBA/Fundação Araucária
- n/a/Financiadora de Estudos e Projetos
- Finance code 001/Coordenação de Aperfeicoamento de Pessoal de Nível Superior
LinkOut - more resources
Full Text Sources

