Gut Microbiota Alleviates Intestinal Injury Induced by Extended Exposure to Light via Inhibiting the Activation of NLRP3 Inflammasome in Broiler Chickens
- PMID: 38928401
- PMCID: PMC11203690
- DOI: 10.3390/ijms25126695
Gut Microbiota Alleviates Intestinal Injury Induced by Extended Exposure to Light via Inhibiting the Activation of NLRP3 Inflammasome in Broiler Chickens
Abstract
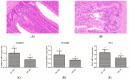
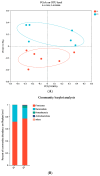
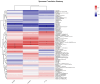
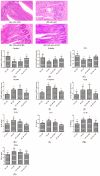
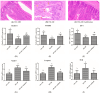
Light pollution is a potential risk for intestinal health in humans and animals. The gut microbiota is associated with the development of intestinal inflammation induced by extended exposure to light, but the underlying mechanism is not yet clear. The results of this study showed that extended exposure to light (18L:6D) damaged intestinal morphology, downregulated the expression of tight junction proteins, and upregulated the expression of the NLRP3 inflammasome and the concentration of pro-inflammatory cytokines. In addition, extended exposure to light significantly decreased the abundance of Lactobacillus, Butyricicoccus, and Sellimonas and increased the abundance of Bifidobacterium, unclassified Oscillospirales, Family_XIII_UCG-001, norank_f__norank_o__Clostridia_vadinBB60_group, and Defluviitaleaceae_UCG-01. Spearman correlation analysis indicated that gut microbiota dysbiosis positively correlated with the activation of the NLRP3 inflammasome. The above results indicated that extended exposure to light induced intestinal injury by NLRP3 inflammasome activation and gut microbiota dysbiosis. Antibiotic depletion intestinal microbiota treatment and cecal microbiota transplantation (CMT) from the 12L:12D group to 18L:6D group indicated that the gut microbiota alleviated intestinal inflammatory injury induced by extended exposure to light via inhibiting the activation of the NLRP3 inflammasome. In conclusion, our findings indicated that the gut microbiota can alleviate intestinal inflammation induced by extended exposure to light via inhibiting the activation of the NLRP3 inflammasome.
Keywords: NLRP3 inflammasome; broiler chickens; extended exposure to light; gut microbiota; intestinal inflammation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
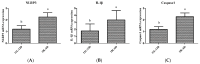
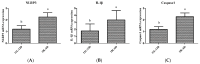


Similar articles
-
Research Note: The effect of photoperiod on the NLRP3 inflammasome and gut microbiota in broiler chickens.Poult Sci. 2024 Apr;103(4):103507. doi: 10.1016/j.psj.2024.103507. Epub 2024 Feb 1. Poult Sci. 2024. PMID: 38387288 Free PMC article.
-
Gegen Qinlian decoction ameliorates TNBS-induced ulcerative colitis by regulating Th2/Th1 and Tregs/Th17 cells balance, inhibiting NLRP3 inflammasome activation and reshaping gut microbiota.J Ethnopharmacol. 2024 Jun 28;328:117956. doi: 10.1016/j.jep.2024.117956. Epub 2024 Feb 29. J Ethnopharmacol. 2024. PMID: 38428658
-
Dietary tryptophan alleviates intestinal inflammation caused by long photoperiod via gut microbiota derived tryptophan metabolites-NLRP3 pathway in broiler chickens.Poult Sci. 2024 Apr;103(4):103509. doi: 10.1016/j.psj.2024.103509. Epub 2024 Jan 29. Poult Sci. 2024. PMID: 38387289 Free PMC article.
-
The crosstalk between NLRP3 inflammasome and gut microbiome in atherosclerosis.Pharmacol Res. 2022 Jul;181:106289. doi: 10.1016/j.phrs.2022.106289. Epub 2022 Jun 6. Pharmacol Res. 2022. PMID: 35671922 Review.
-
The role of the microbiome and the NLRP3 inflammasome in the gut and lung.J Leukoc Biol. 2020 Sep;108(3):925-935. doi: 10.1002/JLB.3MR0720-472RR. Epub 2020 Aug 17. J Leukoc Biol. 2020. PMID: 33405294 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

