A-Syn(ful) MAM: A Fresh Perspective on a Converging Domain in Parkinson's Disease
- PMID: 38928232
- PMCID: PMC11203789
- DOI: 10.3390/ijms25126525
A-Syn(ful) MAM: A Fresh Perspective on a Converging Domain in Parkinson's Disease
Abstract
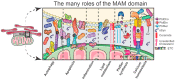
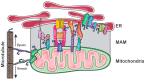
Parkinson's disease (PD) is a disease of an unknown origin. Despite that, decades of research have provided considerable evidence that alpha-synuclein (αSyn) is central to the pathogenesis of disease. Mitochondria-associated endoplasmic reticulum (ER) membranes (MAMs) are functional domains formed at contact sites between the ER and mitochondria, with a well-established function of MAMs being the control of lipid homeostasis within the cell. Additionally, there are numerous proteins localized or enriched at MAMs that have regulatory roles in several different molecular signaling pathways required for cellular homeostasis, such as autophagy and neuroinflammation. Alterations in several of these signaling pathways that are functionally associated with MAMs are found in PD. Taken together with studies that find αSyn localized at MAMs, this has implicated MAM (dys)function as a converging domain relevant to PD. This review will highlight the many functions of MAMs and provide an overview of the literature that finds αSyn, in addition to several other PD-related proteins, localized there. This review will also detail the direct interaction of αSyn and αSyn-interacting partners with specific MAM-resident proteins. In addition, recent studies exploring new methods to investigate MAMs will be discussed, along with some of the controversies regarding αSyn, including its several conformations and subcellular localizations. The goal of this review is to highlight and provide insight on a domain that is incompletely understood and, from a PD perspective, highlight those complex interactions that may hold the key to understanding the pathomechanisms underlying PD, which may lead to the targeted development of new therapeutic strategies.
Keywords: Parkinson’s disease (PD); alpha-synuclein (αSyn); mitochondrial-associated ER membranes (MAMs).
Conflict of interest statement
The author declares no conflict of interest.
Figures
Similar articles
-
Overexpression of α-synuclein inhibits mitochondrial Ca2+ trafficking between the endoplasmic reticulum and mitochondria through MAMs by altering the GRP75-IP3R interaction.J Neurosci Res. 2021 Nov;99(11):2932-2947. doi: 10.1002/jnr.24952. Epub 2021 Sep 12. J Neurosci Res. 2021. PMID: 34510532
-
Phillyrin promotes autophagosome formation in A53T-αSyn-induced Parkinson's disease model via modulation of REEP1.Phytomedicine. 2024 Nov;134:155952. doi: 10.1016/j.phymed.2024.155952. Epub 2024 Aug 18. Phytomedicine. 2024. PMID: 39178680
-
Mitochondria-Associated Membranes (MAMs): Overview and Its Role in Parkinson's Disease.Mol Neurobiol. 2017 Oct;54(8):6287-6303. doi: 10.1007/s12035-016-0140-8. Epub 2016 Oct 6. Mol Neurobiol. 2017. PMID: 27714635 Review.
-
A new role for α-synuclein in Parkinson's disease: Alteration of ER-mitochondrial communication.Mov Disord. 2015 Jul;30(8):1026-33. doi: 10.1002/mds.26239. Epub 2015 May 7. Mov Disord. 2015. PMID: 25952565 Review.
-
Alpha-synuclein-induced mitochondrial dysfunction is mediated via a sirtuin 3-dependent pathway.Mol Neurodegener. 2020 Jan 13;15(1):5. doi: 10.1186/s13024-019-0349-x. Mol Neurodegener. 2020. PMID: 31931835 Free PMC article.
Cited by
-
The Role of Alpha-Synuclein in Synucleinopathy: Impact on Lipid Regulation at Mitochondria-ER Membranes.bioRxiv [Preprint]. 2024 Jun 17:2024.06.17.599406. doi: 10.1101/2024.06.17.599406. bioRxiv. 2024. PMID: 38948777 Free PMC article. Preprint.
References
-
- Hamza T.H., Zabetian C.P., Tenesa A., Laederach A., Montimurro J., Yearout D., Kay D.M., Doheny K.F., Paschall J., Pugh E., et al. Common Genetic Variation in the HLA Region Is Associated with Late-Onset Sporadic Parkinson’s Disease. Nat. Genet. 2010;42:781–785. doi: 10.1038/ng.642. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

