Chlorogenic Acid and Cinnamaldehyde in Combination Inhibit Metastatic Traits and Induce Apoptosis via Akt Downregulation in Breast Cancer Cells
- PMID: 38928123
- PMCID: PMC11204099
- DOI: 10.3390/ijms25126417
Chlorogenic Acid and Cinnamaldehyde in Combination Inhibit Metastatic Traits and Induce Apoptosis via Akt Downregulation in Breast Cancer Cells
Abstract
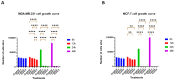
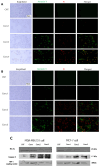
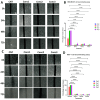
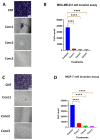
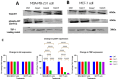
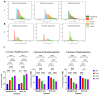
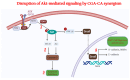
Most reported breast cancer-associated deaths are directly correlated with metastatic disease. Additionally, the primary goal of treating metastatic breast cancer is to prolong life. Thus, there remains the need for more effective and safer strategies to treat metastatic breast cancer. Recently, more attention has been given to natural products (or phytochemicals) as potential anticancer treatments. This study aimed to investigate the synergistic effects of the combination of the phytochemicals chlorogenic acid and cinnamaldehyde (CGA and CA) toward inhibiting metastasis. The hypothesis was that CGA and CA in combination decrease the metastatic potential of breast cancer cells by inhibiting their invasive and migratory abilities as well as the induction of apoptosis via the downregulation of the Akt, disrupting its signal transduction pathway. To test this, wound-healing and Transwell™ Matrigel™ assays were conducted to assess changes in the migration and invasion properties of the cells; apoptosis was analyzed by fluorescence microscopy for Annexin V/propidium iodide; and immunoblotting and FACSort were performed on markers for the epithelial-to-mesenchymal transition status. The results show that CGA and CA significantly downregulated Akt activation by inhibiting phosphorylation. Consequently, increased caspase 3 and decreased Bcl2-α levels were observed, and apoptosis was confirmed. The inhibition of metastatic behavior was demonstrated by the attenuation of N-cadherin, fibronectin, vimentin, and MMP-9 expressions with concomitant increased expressions of E-cadherin and EpCAM. In summary, the present study demonstrated that CGA and CA in combination downregulated Akt activation, inhibited the metastatic potential, and induced apoptosis in different breast cancer cell lines.
Keywords: breast cancer; chlorogenic acid; cinnamaldehyde; metastasis; natural products; phytochemicals.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Chlorogenic acid induces apoptosis, inhibits metastasis and improves antitumor immunity in breast cancer via the NF‑κB signaling pathway.Oncol Rep. 2021 Feb;45(2):717-727. doi: 10.3892/or.2020.7891. Epub 2020 Dec 9. Oncol Rep. 2021. PMID: 33416150 Free PMC article.
-
Chrysin inhibits metastatic potential of human triple-negative breast cancer cells by modulating matrix metalloproteinase-10, epithelial to mesenchymal transition, and PI3K/Akt signaling pathway.J Appl Toxicol. 2014 Jan;34(1):105-12. doi: 10.1002/jat.2941. Epub 2013 Oct 10. J Appl Toxicol. 2014. PMID: 24122885
-
Suppression of growth, migration and invasion of highly-metastatic human breast cancer cells by berbamine and its molecular mechanisms of action.Mol Cancer. 2009 Oct 1;8:81. doi: 10.1186/1476-4598-8-81. Mol Cancer. 2009. PMID: 19796390 Free PMC article.
-
Cinnamaldehyde: Pharmacokinetics, anticancer properties and therapeutic potential (Review).Mol Med Rep. 2024 Sep;30(3):163. doi: 10.3892/mmr.2024.13287. Epub 2024 Jul 12. Mol Med Rep. 2024. PMID: 38994757 Free PMC article. Review.
-
Naturally Occurring Phytochemicals to Target Breast Cancer Cell Signaling.Appl Biochem Biotechnol. 2024 Jul;196(7):4644-4660. doi: 10.1007/s12010-023-04734-0. Epub 2023 Sep 29. Appl Biochem Biotechnol. 2024. PMID: 37773580 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

