C. elegans Germline as Three Distinct Tumor Models
- PMID: 38927305
- PMCID: PMC11200432
- DOI: 10.3390/biology13060425
C. elegans Germline as Three Distinct Tumor Models
Abstract
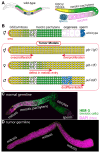
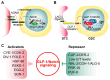
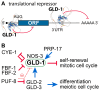
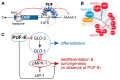
Tumor cells display abnormal growth and division, avoiding the natural process of cell death. These cells can be benign (non-cancerous growth) or malignant (cancerous growth). Over the past few decades, numerous in vitro or in vivo tumor models have been employed to understand the molecular mechanisms associated with tumorigenesis in diverse regards. However, our comprehension of how non-tumor cells transform into tumor cells at molecular and cellular levels remains incomplete. The nematode C. elegans has emerged as an excellent model organism for exploring various phenomena, including tumorigenesis. Although C. elegans does not naturally develop cancer, it serves as a valuable platform for identifying oncogenes and the underlying mechanisms within a live organism. In this review, we describe three distinct germline tumor models in C. elegans, highlighting their associated mechanisms and related regulators: (1) ectopic proliferation due to aberrant activation of GLP-1/Notch signaling, (2) meiotic entry failure resulting from the loss of GLD-1/STAR RNA-binding protein, (3) spermatogenic dedifferentiation caused by the loss of PUF-8/PUF RNA-binding protein. Each model requires the mutations of specific genes (glp-1, gld-1, and puf-8) and operates through distinct molecular mechanisms. Despite these differences in the origins of tumorigenesis, the internal regulatory networks within each tumor model display shared features. Given the conservation of many of the regulators implicated in C. elegans tumorigenesis, it is proposed that these unique models hold significant potential for enhancing our comprehension of the broader control mechanisms governing tumorigenesis.
Keywords: C. elegans germline; GLD-1; GLP-1/Notch signaling; PUF-8; RNA-binding proteins; tumorigenesis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Dynamic Field Theory of Executive Function: Identifying Early Neurocognitive Markers.Monogr Soc Res Child Dev. 2024 Dec;89(3):7-109. doi: 10.1111/mono.12478. Monogr Soc Res Child Dev. 2024. PMID: 39628288 Free PMC article.
-
Far Posterior Approach for Rib Fracture Fixation: Surgical Technique and Tips.JBJS Essent Surg Tech. 2024 Dec 6;14(4):e23.00094. doi: 10.2106/JBJS.ST.23.00094. eCollection 2024 Oct-Dec. JBJS Essent Surg Tech. 2024. PMID: 39650795 Free PMC article.
-
Can a Liquid Biopsy Detect Circulating Tumor DNA With Low-passage Whole-genome Sequencing in Patients With a Sarcoma? A Pilot Evaluation.Clin Orthop Relat Res. 2025 Jan 1;483(1):39-48. doi: 10.1097/CORR.0000000000003161. Epub 2024 Jun 21. Clin Orthop Relat Res. 2025. PMID: 38905450
-
Interventions to reduce harm from continued tobacco use.Cochrane Database Syst Rev. 2016 Oct 13;10(10):CD005231. doi: 10.1002/14651858.CD005231.pub3. Cochrane Database Syst Rev. 2016. PMID: 27734465 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

