Activation of the Wnt/β-catenin signalling pathway enhances exosome production by hucMSCs and improves their capability to promote diabetic wound healing
- PMID: 38926800
- PMCID: PMC11201861
- DOI: 10.1186/s12951-024-02650-x
Activation of the Wnt/β-catenin signalling pathway enhances exosome production by hucMSCs and improves their capability to promote diabetic wound healing
Abstract
Background: The use of stem cell-derived exosomes (Exos) as therapeutic vehicles is receiving increasing attention. Exosome administration has several advantages over cell transplantation, thus making exosomes promising candidates for large-scale clinical implementation and commercialization. However, exosome extraction and purification efficiencies are relatively low, and therapeutic heterogeneity is high due to differences in culture conditions and cell viability. Therefore, in this study, we investigated a priming procedure to enhance the production and therapeutic effects of exosomes from human umbilical cord mesenchymal stem cells (hucMSCs). After preconditioning hucMSCs with agonists/inhibitors that target the Wnt/β-catenin pathway, we assessed both the production of exosomes and the therapeutic efficacy of the optimized exosomes in the context of diabetic wound healing, hoping to provide a safer, more stable and more effective option for clinical application.
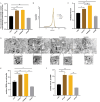
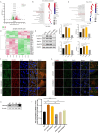
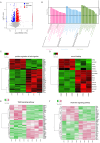
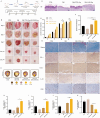
Results: The Wnt signalling pathway agonist CHIR99021 increased exosome production by 1.5-fold without causing obvious changes in the characteristics of the hucMSCs or the size of the exosome particles. Further studies showed that CHIR99021 promoted the production of exosomes by facilitating exocytosis. This process was partly mediated by SNAP25. To further explore whether CHIR99021 changed the cargo that was loaded into the exosomes and its therapeutic effects, we performed proteomic and transcriptomic analyses of exosomes from primed and control hucMSCs. The results showed that CHIR99021 significantly upregulated the expression of proteins that are associated with cell migration and wound healing. Animal experiments confirmed that, compared to control hucMSC-derived exosomes, CHIR99021-pretreated hucMSC-derived exosomes (CHIR-Exos) significantly accelerated wound healing in diabetic mice, enhanced local collagen deposition, promoted angiogenesis, and reduced chronic inflammation. Subsequent in vitro experiments confirmed that the CHIR-Exos promoted wound healing by facilitating cell migration, inhibiting oxidative stress-induced apoptosis, and preventing cell cycle arrest.
Conclusions: The Wnt agonist CHIR99021 significantly increased exosome secretion by hucMSCs, which was partly mediated by SNAP25. Notably, CHIR99021 treatment also significantly increased the exosomal levels of proteins that are associated with wound healing and cell migration, resulting in enhanced acceleration of wound healing. All of these results suggested that pretreatment of hucMSCs with CHIR99021 not only promoted exosome production but also improved the exosome therapeutic efficacy, thus providing a promising option for large-scale clinical implementation and commercialization.
Keywords: Exocytosis; Exosomes; HucMSCs; Wnt/β-catenin signalling; Wound healing.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
miR-100-5p in human umbilical cord mesenchymal stem cell-derived exosomes mediates eosinophilic inflammation to alleviate atherosclerosis via the FZD5/Wnt/β-catenin pathway.Acta Biochim Biophys Sin (Shanghai). 2021 Aug 31;53(9):1166-1176. doi: 10.1093/abbs/gmab093. Acta Biochim Biophys Sin (Shanghai). 2021. PMID: 34254638
-
Exosomes Derived from Human Umbilical Cord Mesenchymal Stem Cells Accelerate Diabetic Wound Healing via Promoting M2 Macrophage Polarization, Angiogenesis, and Collagen Deposition.Int J Mol Sci. 2022 Sep 9;23(18):10421. doi: 10.3390/ijms231810421. Int J Mol Sci. 2022. PMID: 36142334 Free PMC article.
-
Umbilical Cord-Derived Mesenchymal Stem Cell-Derived Exosomes Combined Pluronic F127 Hydrogel Promote Chronic Diabetic Wound Healing and Complete Skin Regeneration.Int J Nanomedicine. 2020 Aug 11;15:5911-5926. doi: 10.2147/IJN.S249129. eCollection 2020. Int J Nanomedicine. 2020. PMID: 32848396 Free PMC article.
-
The Therapeutic Potential of Human Umbilical Cord Mesenchymal Stromal Cells Derived Exosomes for Wound Healing: Harnessing Exosomes as a Cell-free Therapy.J Stem Cells Regen Med. 2024 May 31;20(1):14-23. doi: 10.46582/jsrm.2003003. eCollection 2024. J Stem Cells Regen Med. 2024. PMID: 39044811 Free PMC article. Review.
-
The potential therapeutic effect of human umbilical cord mesenchymal stem cell-derived exosomes in bronchopulmonary dysplasia.Life Sci. 2024 Nov 15;357:123047. doi: 10.1016/j.lfs.2024.123047. Epub 2024 Sep 12. Life Sci. 2024. PMID: 39260518 Review.
References
-
- Li K, Yan G, Huang H, Zheng M, Ma K, Cui X, Lu D, Zheng L, Zhu B, Cheng J, Zhao J. Anti-inflammatory and immunomodulatory effects of the extracellular vesicles derived from human umbilical cord mesenchymal stem cells on osteoarthritis via M2 macrophages. J Nanobiotechnol. 2022;20:38. doi: 10.1186/s12951-021-01236-1. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- 82070800/the National Nature Science Foundation
- ZR2022ZD15/a Special fund for Taishan Industrial leading talent project, Major basic research project of Shandong Natural Science Foundation
- 2022YFA1004801-2/National Key Research and Development Program of China
- 2022-N-02-13/China International Medical Foundation
LinkOut - more resources
Full Text Sources

