Novel Multiparametric Bioelectronic Measurement System for Monitoring Virus-Induced Alterations in Functional Neuronal Networks
- PMID: 38920600
- PMCID: PMC11202209
- DOI: 10.3390/bios14060295
Novel Multiparametric Bioelectronic Measurement System for Monitoring Virus-Induced Alterations in Functional Neuronal Networks
Abstract
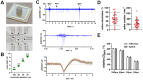
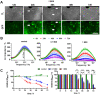
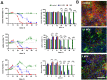
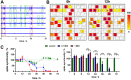
Development and optimisation of bioelectronic monitoring techniques like microelectrode array-based field potential measurement and impedance spectroscopy for the functional, label-free and non-invasive monitoring of in vitro neuronal networks is widely investigated in the field of biosensors. Thus, these techniques were individually used to demonstrate the capabilities of, e.g., detecting compound-induced toxicity in neuronal culture models. In contrast, extended application for investigating the effects of central nervous system infecting viruses are rarely described. In this context, we wanted to analyse the effect of herpesviruses on functional neuronal networks. Therefore, we developed a unique hybrid bioelectronic monitoring platform that allows for performing field potential monitoring and impedance spectroscopy on the same microelectrode. In the first step, a neuronal culture model based on primary hippocampal cells from neonatal rats was established with reproducible and stable synchronised electrophysiological network activity after 21 days of cultivation on microelectrode arrays. For a proof of concept, the pseudorabies model virus PrV Kaplan-ΔgG-GFP was applied and the effect on the neuronal networks was monitored by impedance spectroscopy and field potential measurement for 72 h in a multiparametric mode. Analysis of several bioelectronic parameters revealed a virus concentration-dependent degeneration of the neuronal network within 24-48 h, with a significant early change in electrophysiological activity, subsequently leading to a loss of activity and network synchronicity. In conclusion, we successfully developed a microelectrode array-based hybrid bioelectronic measurement platform for quantitative monitoring of pathologic effects of a herpesvirus on electrophysiological active neuronal networks.
Keywords: field potential monitoring; impedance spectroscopy; microelectrode arrays; primary hippocampal neurons; pseudorabies virus model.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Impedimetric real-time monitoring of neural pluripotent stem cell differentiation process on microelectrode arrays.Biosens Bioelectron. 2016 Dec 15;86:277-286. doi: 10.1016/j.bios.2016.06.056. Epub 2016 Jun 20. Biosens Bioelectron. 2016. PMID: 27387257
-
An in vitro system to study trans-neuronal spread of pseudorabies virus infection.Vet Microbiol. 2006 Mar 31;113(3-4):193-7. doi: 10.1016/j.vetmic.2005.11.010. Epub 2005 Dec 1. Vet Microbiol. 2006. PMID: 16326047
-
Comprehensive human stem cell differentiation in a 2D and 3D mode to cardiomyocytes for long-term cultivation and multiparametric monitoring on a multimodal microelectrode array setup.Biosens Bioelectron. 2019 Feb 1;126:624-631. doi: 10.1016/j.bios.2018.10.061. Epub 2018 Oct 29. Biosens Bioelectron. 2019. PMID: 30508787
-
[Studies on neuronal tracing with pseudorabies virus].Bing Du Xue Bao. 2014 May;30(3):333-7. Bing Du Xue Bao. 2014. PMID: 25118391 Review. Chinese.
-
Practical considerations for the use of pseudorabies virus in transneuronal studies of neural circuitry.Neurosci Biobehav Rev. 1998 Oct;22(6):685-94. doi: 10.1016/s0149-7634(98)00007-4. Neurosci Biobehav Rev. 1998. PMID: 9809304 Review.
References
-
- Yolken R.H., Jones-Brando L., Dunigan D.D., Kannan G., Dickerson F., Severance E., Sabunciyan S., Talbot C.C., Jr., Prandovszky E., Gurnon J.R., et al. Chlorovirus ATCV-1 is part of the human oropharyngeal virome and is associated with changes in cognitive functions in humans and mice. Proc. Natl. Acad. Sci. USA. 2014;111:16106–16111. doi: 10.1073/pnas.1418895111. - DOI - PMC - PubMed
-
- Ghassemi S., Asgari T., Mirzapour-Delavar H., Aliakbari S., Pourbadie H.G., Prehaud C., Lafon M., Gholami A., Azadmanesh K., Naderi N., et al. Lentiviral Expression of Rabies Virus Glycoprotein in the Rat Hippocampus Strengthens Synaptic Plasticity. Cell. Mol. Neurobiol. 2021;42:1429–1440. doi: 10.1007/s10571-020-01032-9. - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

