The tyrosine kinase KDR is essential for the survival of HTLV-1-infected T cells by stabilizing the Tax oncoprotein
- PMID: 38918393
- PMCID: PMC11199648
- DOI: 10.1038/s41467-024-49737-5
The tyrosine kinase KDR is essential for the survival of HTLV-1-infected T cells by stabilizing the Tax oncoprotein
Abstract
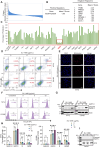
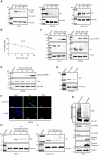
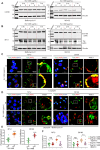
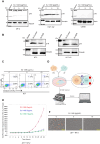
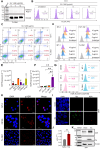
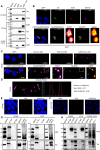
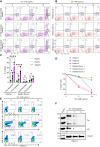
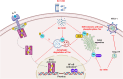
Human T-cell leukemia virus type 1 (HTLV-1) infection is linked to the development of adult T-cell leukemia/lymphoma (ATLL) and the neuroinflammatory disease, HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP). The HTLV-1 Tax oncoprotein regulates viral gene expression and persistently activates NF-κB to maintain the viability of HTLV-1-infected T cells. Here, we utilize a kinome-wide shRNA screen to identify the tyrosine kinase KDR as an essential survival factor of HTLV-1-transformed cells. Inhibition of KDR specifically induces apoptosis of Tax expressing HTLV-1-transformed cell lines and CD4 + T cells from HAM/TSP patients. Furthermore, inhibition of KDR triggers the autophagic degradation of Tax resulting in impaired NF-κB activation and diminished viral transmission in co-culture assays. Tax induces the expression of KDR, forms a complex with KDR, and is phosphorylated by KDR. These findings suggest that Tax stability is dependent on KDR activity which could be exploited as a strategy to target Tax in HTLV-1-associated diseases.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
HTLV-1 Tax and adult T-cell leukemia.Front Biosci. 2007 Jan 1;12:1496-507. doi: 10.2741/2163. Front Biosci. 2007. PMID: 17127397 Review.
-
The E3/E4 ubiquitin conjugation factor UBE4B interacts with and ubiquitinates the HTLV-1 Tax oncoprotein to promote NF-κB activation.PLoS Pathog. 2020 Dec 23;16(12):e1008504. doi: 10.1371/journal.ppat.1008504. eCollection 2020 Dec. PLoS Pathog. 2020. PMID: 33362245 Free PMC article.
-
Role of the HTLV-1 viral factors in the induction of apoptosis.Biomed Pharmacother. 2017 Jan;85:334-347. doi: 10.1016/j.biopha.2016.11.034. Epub 2016 Nov 23. Biomed Pharmacother. 2017. PMID: 27887847 Review.
-
Suppression of Type I Interferon Production by Human T-Cell Leukemia Virus Type 1 Oncoprotein Tax through Inhibition of IRF3 Phosphorylation.J Virol. 2016 Mar 28;90(8):3902-3912. doi: 10.1128/JVI.00129-16. Print 2016 Apr. J Virol. 2016. PMID: 26819312 Free PMC article.
-
Cell surface markers in HTLV-1 pathogenesis.Viruses. 2011 Aug;3(8):1439-59. doi: 10.3390/v3081439. Epub 2011 Aug 16. Viruses. 2011. PMID: 21994790 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

