Deregulation of oxidative phosphorylation pathways in embryos derived in vitro from prepubertal and pubertal heifers based on whole-transcriptome sequencing
- PMID: 38914933
- PMCID: PMC11197288
- DOI: 10.1186/s12864-024-10532-7
Deregulation of oxidative phosphorylation pathways in embryos derived in vitro from prepubertal and pubertal heifers based on whole-transcriptome sequencing
Abstract
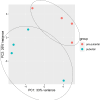
Background: Although, oocytes from prepubertal donors are known to be less developmentally competent than those from adult donors it does not restrain their ability to produce full-term pregnancies. The transcriptomic profile of embryos could be used as a predictor for embryo's individual developmental competence. The aim of the study was to compare transcriptomic profile of blastocysts derived from prepubertal and pubertal heifers oocytes. Bovine cumulus-oocyte complexes (COCs) were obtained by ovum pick- up method from prepubertal and pubertal heifers. After in vitro maturation COCs were fertilized and cultured to the blastocyst stage. Total RNA was isolated from both groups of blastocysts and RNA-seq was performed. Gene ontology analysis was performed by DAVID (Database for Annotation, Visualization and Integrated Discovery).
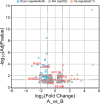
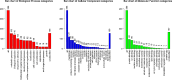
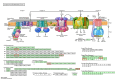
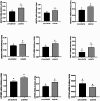
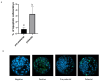
Results: A higher average blastocyst rate was obtained in the pubertal than in the pre-pubertal group. There were no differences in the quality of blastocysts between the examined groups. We identified 436 differentially expressed genes (DEGs) between blastocysts derived from researched groups, of which 247 DEGs were downregulated in blastocysts derived from pubertal compared to prepubertal heifers oocytes, and 189 DEGs were upregulated. The genes involved in mitochondrial function, including oxidative phosphorylation (OXPHOS) were found to be different in studied groups using Kyoto Encyclopedia of Genes (KEGG) pathway analysis and 8 of those DEGs were upregulated and 1 was downregulated in blastocysts derived from pubertal compared to prepubertal heifers oocytes. DEGs associated with mitochondrial function were found: ATP synthases (ATP5MF-ATP synthase membrane subunit f, ATP5PD- ATP synthase peripheral stalk subunit d, ATP12A- ATPase H+/K + transporting non-gastric alpha2 subunit), NADH dehydrogenases (NDUFS3- NADH: ubiquinone oxidoreductase subunit core subunit S3, NDUFA13- NADH: ubiquinone oxidoreductase subunit A13, NDUFA3- NADH: ubiquinone oxidoreductase subunit A3), cytochrome c oxidase (COX17), cytochrome c somatic (CYCS) and ubiquinol cytochrome c reductase core protein 1 (UQCRC1). We found lower number of apoptotic cells in blastocysts derived from oocytes collected from prepubertal than those obtained from pubertal donors.
Conclusions: Despite decreased expression of genes associated with OXPHOS pathway in blastocysts from prepubertal heifers oocytes, the increased level of ATP12A together with the lower number of apoptotic cells in these blastocysts might support their survival after transfer.
Keywords: Blastocyst; Cow; Mitochondria; Next-generation sequencing; Prepubertal heifer.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Mitochondrial DNA content and developmental competence of blastocysts derived from pre-pubertal heifer oocytes.Theriogenology. 2022 Oct 1;191:207-220. doi: 10.1016/j.theriogenology.2022.07.017. Epub 2022 Aug 8. Theriogenology. 2022. PMID: 35998404
-
Melatonin enhances in vitro developmental competence of cumulus-oocyte complexes collected by ovum pick-up in prepubertal and adult dairy cattle.Theriogenology. 2021 Feb;161:285-293. doi: 10.1016/j.theriogenology.2020.12.011. Epub 2020 Dec 11. Theriogenology. 2021. PMID: 33360610
-
The global gene expression outline of the bovine blastocyst: reflector of environmental conditions and predictor of developmental capacity.BMC Genomics. 2021 Jun 3;22(1):408. doi: 10.1186/s12864-021-07693-0. BMC Genomics. 2021. PMID: 34082721 Free PMC article.
-
Prepubertal goat oocytes from large follicles result in similar blastocyst production and embryo ploidy than those from adult goats.Theriogenology. 2011 Jul 1;76(1):1-11. doi: 10.1016/j.theriogenology.2010.12.014. Epub 2011 Feb 4. Theriogenology. 2011. PMID: 21295839 Review.
-
Effects of in vivo prematuration and in vivo final maturation on developmental capacity and quality of pre-implantation embryos.Theriogenology. 2002 Jan 1;57(1):5-20. doi: 10.1016/s0093-691x(01)00655-0. Theriogenology. 2002. PMID: 11775980 Review.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

