Evidence that the cold- and menthol-sensing functions of the human TRPM8 channel evolved separately
- PMID: 38905339
- PMCID: PMC11192081
- DOI: 10.1126/sciadv.adm9228
Evidence that the cold- and menthol-sensing functions of the human TRPM8 channel evolved separately
Abstract
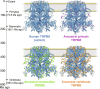
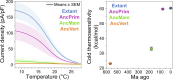
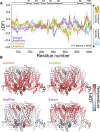
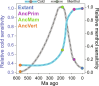
Transient receptor potential melastatin 8 (TRPM8) is a temperature- and menthol-sensitive ion channel that contributes to diverse physiological roles, including cold sensing and pain perception. Clinical trials targeting TRPM8 have faced repeated setbacks predominantly due to the knowledge gap in unraveling the molecular underpinnings governing polymodal activation. A better understanding of the molecular foundations between the TRPM8 activation modes may aid the development of mode-specific, thermal-neutral therapies. Ancestral sequence reconstruction was used to explore the origins of TRPM8 activation modes. By resurrecting key TRPM8 nodes along the human evolutionary trajectory, we gained valuable insights into the trafficking, stability, and function of these ancestral forms. Notably, this approach unveiled the differential emergence of cold and menthol sensitivity over evolutionary time, providing a fresh perspective on complex polymodal behavior. These studies provide a paradigm for understanding polymodal behavior in TRPM8 and other proteins with the potential to enhance our understanding of sensory receptor biology and pave the way for innovative therapeutic interventions.
Figures


Similar articles
-
Intimacies and physiological role of the polymodal cold-sensitive ion channel TRPM8.Curr Top Membr. 2014;74:293-324. doi: 10.1016/B978-0-12-800181-3.00011-7. Curr Top Membr. 2014. PMID: 25366241 Review.
-
Two different avian cold-sensitive sensory neurons: Transient receptor potential melastatin 8 (TRPM8)-dependent and -independent activation mechanisms.Neuropharmacology. 2016 Dec;111:130-141. doi: 10.1016/j.neuropharm.2016.08.039. Epub 2016 Aug 31. Neuropharmacology. 2016. PMID: 27590914
-
Bidirectional modulation of thermal and chemical sensitivity of TRPM8 channels by the initial region of the N-terminal domain.J Biol Chem. 2014 Aug 8;289(32):21828-43. doi: 10.1074/jbc.M114.565994. Epub 2014 Jun 10. J Biol Chem. 2014. PMID: 24917670 Free PMC article.
-
The roles of iPLA2, TRPM8 and TRPA1 in chemically induced cold hypersensitivity.Mol Pain. 2010 Jan 21;6:4. doi: 10.1186/1744-8069-6-4. Mol Pain. 2010. PMID: 20092626 Free PMC article.
-
TRPM8 Channels: Advances in Structural Studies and Pharmacological Modulation.Int J Mol Sci. 2021 Aug 7;22(16):8502. doi: 10.3390/ijms22168502. Int J Mol Sci. 2021. PMID: 34445208 Free PMC article. Review.
References
-
- McKemy D. D., Neuhausser W. M., Julius D., Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 416, 52–58 (2002). - PubMed
-
- Peier A. M., Moqrich A., Hergarden A. C., Reeve A. J., Andersson D. A., Story G. M., Earley T. J., Dragoni I., McIntyre P., Bevan S., Patapoutian A., A TRP channel that senses cold stimuli and menthol. Cell 108, 705–715 (2002). - PubMed
-
- Hensel H., Zotterman Y., The effect of menthol on the thermoreceptors. Acta Physiol. Scand. 24, 27–34 (1951). - PubMed
-
- Winchester W. J., Gore K., Glatt S., Petit W., Gardiner J. C., Conlon K., Postlethwaite M., Saintot P. P., Roberts S., Gosset J. R., Matsuura T., Andrews M. D., Glossop P. A., Palmer M. J., Clear N., Collins S., Beaumont K., Reynolds D. S., Inhibition of TRPM8 channels reduces pain in the cold pressor test in humans. J Pharmacol Exp Ther. 351, 259–269 (2014). - PubMed
-
- Horne D. B., Biswas K., Brown J., Bartberger M. D., Clarine J., Davis C. D., Gore V. K., Harried S., Horner M., Kaller M. R., Lehto S. G., Liu Q., Ma V. V., Monenschein H., Nguyen T. T., Yuan C. C., Youngblood B. D., Zhang M., Zhong W., Allen J. R., Chen J. J., Gavva N. R., Discovery of TRPM8 Antagonist (S)-6-(((3-fluoro-4-(trifluoromethoxy)phenyl)(3-fluoropyridin-2-yl)methyl)carbamoyl)nicotinic acid (AMG 333), a clinical candidate for the treatment of migraine. J. Med. Chem. 61, 8186–8201 (2018). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

