Rotating magnetic field improved cognitive and memory impairments in a sporadic ad model of mice by regulating microglial polarization
- PMID: 38904930
- PMCID: PMC11493917
- DOI: 10.1007/s11357-024-01223-y
Rotating magnetic field improved cognitive and memory impairments in a sporadic ad model of mice by regulating microglial polarization
Abstract
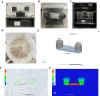
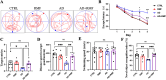
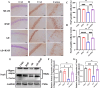
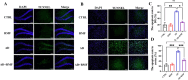
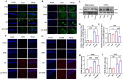
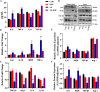
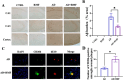
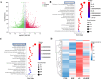
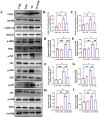
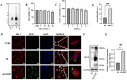
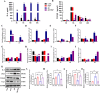
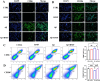
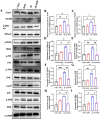
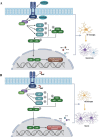
Neuroinflammation, triggered by aberrantly activated microglia, is widely recognized as a key contributor to the initiation and progression of Alzheimer's disease (AD). Microglial activation in the central nervous system (CNS) can be classified into two distinct phenotypes: the pro-inflammatory M1 phenotype and the anti-inflammatory M2 phenotype. In this study, we investigated the effects of a non-invasive rotating magnetic field (RMF) (0.2T, 4Hz) on cognitive and memory impairments in a sporadic AD model of female Kunming mice induced by AlCl3 and D-gal. Our findings revealed significant improvements in cognitive and memory impairments following RMF treatment. Furthermore, RMF treatment led to reduced amyloid-beta (Aβ) deposition, mitigated damage to hippocampal morphology, prevented synaptic and neuronal loss, and alleviated cell apoptosis in the hippocampus and cortex of AD mice. Notably, RMF treatment ameliorated neuroinflammation, facilitated the transition of microglial polarization from M1 to M2, and inhibited the NF-кB/MAPK pathway. Additionally, RMF treatment resulted in reduced aluminum deposition in the brains of AD mice. In cellular experiments, RMF promoted the M1-M2 polarization transition and enhanced amyloid phagocytosis in cultured BV2 cells while inhibiting the TLR4/NF-кB/MAPK pathway. Collectively, these results demonstrate that RMF improves memory and cognitive impairments in a sporadic AD model, potentially by promoting the M1 to M2 transition of microglial polarization through inhibition of the NF-кB/MAPK signaling pathway. These findings suggest the promising therapeutic applications of RMF in the clinical treatment of AD.
Keywords: Microglia polarization; Neuroinflammation; Rotating magnetic field; Sporadic Alzheimer’s disease.
© 2024. The Author(s), under exclusive licence to American Aging Association.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Low-Dose Ionizing Radiation Modulates Microglia Phenotypes in the Models of Alzheimer's Disease.Int J Mol Sci. 2020 Jun 25;21(12):4532. doi: 10.3390/ijms21124532. Int J Mol Sci. 2020. PMID: 32630597 Free PMC article.
-
M2 Macrophage Transplantation Ameliorates Cognitive Dysfunction in Amyloid-β-Treated Rats Through Regulation of Microglial Polarization.J Alzheimers Dis. 2016 Mar 16;52(2):483-95. doi: 10.3233/JAD-151090. J Alzheimers Dis. 2016. PMID: 27003214
-
Rotating magnetic field inhibits Aβ protein aggregation and alleviates cognitive impairment in Alzheimer's disease mice.Zool Res. 2024 Jul 18;45(4):924-936. doi: 10.24272/j.issn.2095-8137.2024.034. Zool Res. 2024. PMID: 39021081 Free PMC article.
-
Myricetin improves pathological changes in 3×Tg-AD mice by regulating the mitochondria-NLRP3 inflammasome-microglia channel by targeting P38 MAPK signaling pathway.Phytomedicine. 2023 Jul;115:154801. doi: 10.1016/j.phymed.2023.154801. Epub 2023 Apr 6. Phytomedicine. 2023. PMID: 37086707
-
Microglial modulation as a therapeutic strategy in Alzheimer's disease: Focus on microglial preconditioning approaches.J Cell Mol Med. 2024 Aug;28(15):e18554. doi: 10.1111/jcmm.18554. J Cell Mol Med. 2024. PMID: 39103747 Free PMC article. Review.
References
-
- Alzheimer’s disease facts and figures. Alzheimers Dement. 2023;19:1598-1695. 10.1002/alz.13016. - PubMed
-
- Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology. 1992;42:631–9. 10.1212/wnl.42.3.631. - PubMed
-
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–8. 10.1126/science.1110647. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

