SMARCB1 Gene Therapy Using a Novel Tumor-Targeted Nanomedicine Enhances Anti-Cancer Efficacy in a Mouse Model of Atypical Teratoid Rhabdoid Tumors
- PMID: 38895149
- PMCID: PMC11185260
- DOI: 10.2147/IJN.S458323
SMARCB1 Gene Therapy Using a Novel Tumor-Targeted Nanomedicine Enhances Anti-Cancer Efficacy in a Mouse Model of Atypical Teratoid Rhabdoid Tumors
Abstract
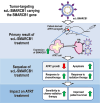
Purpose: Atypical teratoid rhabdoid tumor (ATRT) is a deadly, fast-growing form of pediatric brain cancer with poor prognosis. Most ATRTs are associated with inactivation of SMARCB1, a subunit of the chromatin remodeling complex, which is involved in developmental processes. The recent identification of SMARCB1 as a tumor suppressor gene suggests that restoration of SMARCB1 could be an effective therapeutic approach.
Methods: We tested SMARCB1 gene therapy in SMARCB1-deficient rhabdoid tumor cells using a novel tumor-targeted nanomedicine (termed scL-SMARCB1) to deliver wild-type SMARCB1. Our nanomedicine is a systemically administered immuno-lipid nanoparticle that can actively cross the blood-brain barrier via transferrin receptor-mediated transcytosis and selectively target tumor cells via transferrin receptor-mediated endocytosis. We studied the antitumor activity of the scL-SMARCB1 nanocomplex either as a single agent or in combination with traditional treatment modalities in preclinical models of SMARCB1-deficient ATRT.
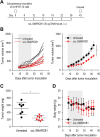
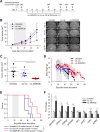
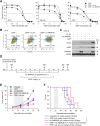
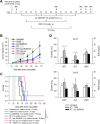
Results: Restoration of SMARCB1 expression by the scL-SMARCB1 nanocomplex blocked proliferation, and induced senescence and apoptosis in ATRT cells. Systemic administration of the scL-SMARCB1 nanocomplex demonstrated antitumor efficacy as monotherapy in mice bearing ATRT xenografts, where the expression of exogenous SMARCB1 modulates MYC-target genes. scL-SMARCB1 demonstrated even greater antitumor efficacy when combined with either cisplatin-based chemotherapy or radiation therapy, resulting in significantly improved survival of ATRT-bearing mice.
Conclusion: Collectively, our data suggest that restoring SMARCB1 function via the scL-SMARCB1 nanocomplex may lead to therapeutic benefits in ATRT patients when combined with traditional chemoradiation therapies.
Keywords: SMARCB1; atypical teratoid rhabdoid tumor; gene therapy; lipid nanoparticle; nanodelivery.
© 2024 Kim et al.
Conflict of interest statement
E.H.C. is one of the inventors of the described technology, for which several patents owned by Georgetown University have been issued. The patents were licensed to SynerGene Therapeutics, Inc. for commercial development. E.H.C. has an equity interest in SynerGene Therapeutics, Inc., and E.H.C. and A.R. serve as paid scientific consultants for SynerGene Therapeutics, Inc. S.S.K. is a salaried employee of SynerGene Therapeutics, Inc. M.M. is a graduate student who was supported by a research agreement between Georgetown University and SynerGene Therapeutics, Inc. J.B.H. serves as the salaried President & CEO of SynerGene Therapeutics, Inc., and owns stock in the same. The authors report no other conflicts of interest in this work.
Figures
Similar articles
-
Inhibition of MYC attenuates tumor cell self-renewal and promotes senescence in SMARCB1-deficient Group 2 atypical teratoid rhabdoid tumors to suppress tumor growth in vivo.Int J Cancer. 2019 Apr 15;144(8):1983-1995. doi: 10.1002/ijc.31873. Epub 2019 Jan 10. Int J Cancer. 2019. PMID: 30230537
-
Functional relevance of genes predicted to be affected by epigenetic alterations in atypical teratoid/rhabdoid tumors.J Neurooncol. 2019 Jan;141(1):43-55. doi: 10.1007/s11060-018-03018-6. Epub 2018 Nov 16. J Neurooncol. 2019. PMID: 30446899
-
Atypical teratoid/rhabdoid tumors (ATRTs) with SMARCA4 mutation are molecularly distinct from SMARCB1-deficient cases.Acta Neuropathol. 2021 Feb;141(2):291-301. doi: 10.1007/s00401-020-02250-7. Epub 2020 Dec 17. Acta Neuropathol. 2021. PMID: 33331994 Free PMC article.
-
Atypical Teratoid/Rhabdoid Sellar Tumor in an Adult with a Familial History of a Germline SMARCB1 Mutation: Case Report and Review of the Literature.World Neurosurg. 2019 Jul;127:336-345. doi: 10.1016/j.wneu.2019.04.083. Epub 2019 Apr 17. World Neurosurg. 2019. PMID: 31004861 Review.
-
Atypical teratoid rhabdoid tumor: molecular insights and translation to novel therapeutics.J Neurooncol. 2020 Oct;150(1):47-56. doi: 10.1007/s11060-020-03639-w. Epub 2020 Oct 6. J Neurooncol. 2020. PMID: 33021733 Free PMC article. Review.
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. - PubMed
-
- Lafay-Cousin L, Hawkins C, Carret AS, et al. Central nervous system atypical teratoid rhabdoid tumours: the Canadian Paediatric Brain Tumour Consortium experience. Eur J Cancer. 2012;48(3):353–359. - PubMed
-
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

