Atractylodes macrocephala Koidz Alleviates Symptoms in Zymosan-Induced Irritable Bowel Syndrome Mouse Model through TRPV1, NaV1.5, and NaV1.7 Channel Modulation
- PMID: 38892616
- PMCID: PMC11174792
- DOI: 10.3390/nu16111683
Atractylodes macrocephala Koidz Alleviates Symptoms in Zymosan-Induced Irritable Bowel Syndrome Mouse Model through TRPV1, NaV1.5, and NaV1.7 Channel Modulation
Abstract
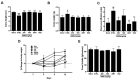
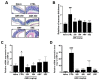
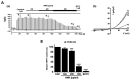
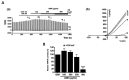
(1) Background: Irritable bowel syndrome (IBS) is a common disease in the gastrointestinal (GI) tract. Atractylodes macrocephala Koidz (AMK) is known as one of the traditional medicines that shows a good efficacy in the GI tract. (2) Methods: We investigated the effect of AMK in a network pharmacology and zymosan-induced IBS animal model. In addition, we performed electrophysiological experiments to confirm the regulatory mechanisms related to IBS. (3) Results: Various characteristics of AMK were investigated using TCMSP data and various analysis systems. AMK restored the macroscopic changes and weight to normal. Colonic mucosa and inflammatory factors were reduced. These effects were similar to those of amitriptyline and sulfasalazine. In addition, transient receptor potential (TRP) V1, voltage-gated Na+ (NaV) 1.5, and NaV1.7 channels were inhibited. (4) Conclusion: These results suggest that AMK may be a promising therapeutic candidate for IBS management through the regulation of ion channels.
Keywords: gastrointestinal disease; ion channel; quality of life; traditional medicine; visceral hypersensitivity.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
The traditional herbal medicines mixture, Banhasasim-tang, relieves the symptoms of irritable bowel syndrome via modulation of TRPA1, NaV1.5 and NaV1.7 channels.J Ethnopharmacol. 2023 Aug 10;312:116499. doi: 10.1016/j.jep.2023.116499. Epub 2023 Apr 13. J Ethnopharmacol. 2023. PMID: 37059250
-
Atractylodes macrocephala Koidz promotes intestinal epithelial restitution via the polyamine--voltage-gated K+ channel pathway.J Ethnopharmacol. 2014 Feb 27;152(1):163-72. doi: 10.1016/j.jep.2013.12.049. Epub 2014 Jan 10. J Ethnopharmacol. 2014. PMID: 24417867
-
Alleviation of Irritable Bowel Syndrome-Like Symptoms and Control of Gut and Brain Responses with Oral Administration of Dolichos lablab L. in a Mouse Model.Nutrients. 2018 Oct 10;10(10):1475. doi: 10.3390/nu10101475. Nutrients. 2018. PMID: 30309025 Free PMC article.
-
Recent advances in pharmacological research on the management of irritable bowel syndrome.Arch Pharm Res. 2018 Oct;41(10):955-966. doi: 10.1007/s12272-018-1068-5. Epub 2018 Aug 21. Arch Pharm Res. 2018. PMID: 30132170 Review.
-
Ion channels, ion channel receptors, and visceral hypersensitivity in irritable bowel syndrome.Neurogastroenterol Motil. 2016 Nov;28(11):1613-1618. doi: 10.1111/nmo.12979. Neurogastroenterol Motil. 2016. PMID: 27781369 Free PMC article. Review.
References
-
- Wang Z., Li R.L., Xu S.F., Chen W.W. Effects of Atractylodes macrocephala monosaccharide composition on cytodifferentiation and villin expression of IEC-6 cells in vitro. J. Chin. Med. Mater. 2010;33:938–944. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

