Host Cell Death and Modulation of Immune Response against Mycobacterium tuberculosis Infection
- PMID: 38892443
- PMCID: PMC11172987
- DOI: 10.3390/ijms25116255
Host Cell Death and Modulation of Immune Response against Mycobacterium tuberculosis Infection
Abstract
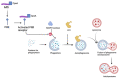
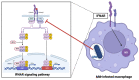
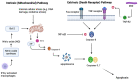
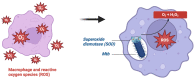
Mycobacterium tuberculosis (Mtb) is the causative agent of tuberculosis (TB), a prevalent infectious disease affecting populations worldwide. A classic trait of TB pathology is the formation of granulomas, which wall off the pathogen, via the innate and adaptive immune systems. Some key players involved include tumor necrosis factor-alpha (TNF-α), foamy macrophages, type I interferons (IFNs), and reactive oxygen species, which may also show overlap with cell death pathways. Additionally, host cell death is a primary method for combating and controlling Mtb within the body, a process which is influenced by both host and bacterial factors. These cell death modalities have distinct molecular mechanisms and pathways. Programmed cell death (PCD), encompassing apoptosis and autophagy, typically confers a protective response against Mtb by containing the bacteria within dead macrophages, facilitating their phagocytosis by uninfected or neighboring cells, whereas necrotic cell death benefits the pathogen, leading to the release of bacteria extracellularly. Apoptosis is triggered via intrinsic and extrinsic caspase-dependent pathways as well as caspase-independent pathways. Necrosis is induced via various pathways, including necroptosis, pyroptosis, and ferroptosis. Given the pivotal role of host cell death pathways in host defense against Mtb, therapeutic agents targeting cell death signaling have been investigated for TB treatment. This review provides an overview of the diverse mechanisms underlying Mtb-induced host cell death, examining their implications for host immunity. Furthermore, it discusses the potential of targeting host cell death pathways as therapeutic and preventive strategies against Mtb infection.
Keywords: Mycobacterium tuberculosis; apoptosis; autophagy; cell death; necrosis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Different modalities of host cell death and their impact on Mycobacterium tuberculosis infection.Am J Physiol Cell Physiol. 2022 Nov 1;323(5):C1444-C1474. doi: 10.1152/ajpcell.00246.2022. Epub 2022 Oct 3. Am J Physiol Cell Physiol. 2022. PMID: 36189975 Free PMC article. Review.
-
Cell death at the cross roads of host-pathogen interaction in Mycobacterium tuberculosis infection.Tuberculosis (Edinb). 2018 Dec;113:99-121. doi: 10.1016/j.tube.2018.09.007. Epub 2018 Sep 29. Tuberculosis (Edinb). 2018. PMID: 30514519 Review.
-
Ursolic Acid Promotes Autophagy by Inhibiting Akt/mTOR and TNF-α/TNFR1 Signaling Pathways to Alleviate Pyroptosis and Necroptosis in Mycobacterium tuberculosis-Infected Macrophages.Inflammation. 2023 Oct;46(5):1749-1763. doi: 10.1007/s10753-023-01839-w. Epub 2023 May 22. Inflammation. 2023. PMID: 37212951
-
Tuberculosis and the art of macrophage manipulation.Pathog Dis. 2018 Jun 1;76(4):fty037. doi: 10.1093/femspd/fty037. Pathog Dis. 2018. PMID: 29762680 Free PMC article. Review.
-
TLR2 is non-redundant in the population and subpopulation responses to Mycobacterium tuberculosis in macrophages and in vivo.mSystems. 2023 Aug 31;8(4):e0005223. doi: 10.1128/msystems.00052-23. Epub 2023 Jul 13. mSystems. 2023. PMID: 37439558 Free PMC article.
Cited by
-
Neisseria meningitidis activates pyroptotic pathways in a mouse model of meningitis: role of a two-partner secretion system.Front Cell Infect Microbiol. 2024 Sep 23;14:1384072. doi: 10.3389/fcimb.2024.1384072. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39376663 Free PMC article.
References
-
- World Health Organization Global Tuberculosis Report 2023. 2023. [(accessed on 12 February 2024)]. Available online: https://www.who.int/publications/i/item/9789240083851.
-
- World Health Organization Global Tuberculosis Report 2021. 2021. [(accessed on 12 February 2024)]. Available online: https://www.who.int/publications/i/item/9789240037021.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

