Transcriptomic Profiling of Sugarcane White Leaf (SCWL) Canes during Maturation Phase
- PMID: 38891358
- PMCID: PMC11174868
- DOI: 10.3390/plants13111551
Transcriptomic Profiling of Sugarcane White Leaf (SCWL) Canes during Maturation Phase
Abstract
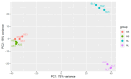
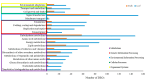
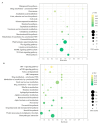
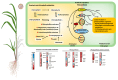
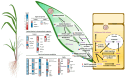
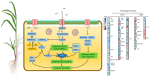
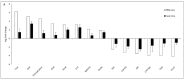
Sugarcane white leaf (SCWL) disease, caused by Candidatus Phytoplasma sacchari, results in the most damage to sugarcane plantations. Some SCWL canes can grow unnoticed through the maturation phase, subsequently resulting in an overall low sugar yield, or they can be used accidentally as seed canes. In this work, 12-month-old SCWL and asymptomatic canes growing in the same field were investigated. An abundance of phytoplasma in SCWL canes affected growth and sugar content as well as alterations of transcriptomic profiles corresponding to several pathways that responded to the infection. Suppression of photosynthesis, porphyrin and chlorophyll metabolism, coupled with an increase in the expression of chlorophyllase, contributed to the reduction in chlorophyll levels and photosynthesis. Blockage of sucrose transport plausibly occurred due to the expression of sugar transporters in leaves but suppression in stalks, resulting in low sugar content in canes. Increased expression of genes associated with MAPK cascades, plant hormone signaling transduction, callose plug formation, the phenylpropanoid pathway, and calcium cascades positively promoted defense mechanisms against phytoplasma colonization by an accumulation of lignin and calcium in response to plant immunity. Significant downregulation of CPK plausibly results in a reduction in antioxidant enzymes and likely facilitates pathogen invasion, while expression of sesquiterpene biosynthesis possibly attracts the insect vectors for transmission, thereby enabling the spread of phytoplasma. Moreover, downregulation of flavonoid biosynthesis potentially intensifies the symptoms of SCWL upon challenge by phytoplasma. These SCWL sugarcane transcriptomic profiles describe the first comprehensive sugarcane-phytoplasma interaction during the harvesting stage. Understanding molecular mechanisms will allow for sustainable management and the prevention of SCWL disease-a crucial benefit to the sugar industry.
Keywords: biotic stress; phytoplasma; plant defense response; sugarcane white leaf; transcriptome.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Survey of Incidence and Nested PCR Detection of Sugarcane White Leaf in Different Varieties.Plant Dis. 2020 Oct;104(10):2665-2668. doi: 10.1094/PDIS-11-19-2482-RE. Epub 2020 Aug 4. Plant Dis. 2020. PMID: 32749946
-
Complete genome sequence of "Candidatus Phytoplasma sacchari" obtained using a filter-based DNA enrichment method and Nanopore sequencing.Front Microbiol. 2023 Oct 2;14:1252709. doi: 10.3389/fmicb.2023.1252709. eCollection 2023. Front Microbiol. 2023. PMID: 37849920 Free PMC article.
-
Alteration in the Stylet Probing Behavior and Host Preference of the Vector Matsumuratettix hiroglyphicus (Hemiptera: Cicadellidae) After Infection with Sugarcane White Leaf Phytoplasma.J Econ Entomol. 2021 Jun 11;114(3):1081-1090. doi: 10.1093/jee/toab059. J Econ Entomol. 2021. PMID: 33822114
-
Transcriptome profiling analysis revealed co-regulation of multiple pathways in jujube during infection by 'Candidatus Phytoplasma ziziphi'.Gene. 2018 Jul 30;665:82-95. doi: 10.1016/j.gene.2018.04.070. Epub 2018 Apr 27. Gene. 2018. PMID: 29709641
-
Post-harvest biology and recent advances of storage technologies in sugarcane.Biotechnol Rep (Amst). 2022 Jan 30;33:e00705. doi: 10.1016/j.btre.2022.e00705. eCollection 2022 Mar. Biotechnol Rep (Amst). 2022. PMID: 35145888 Free PMC article. Review.
Cited by
-
Interaction between Sugarcane and Environmental Stressors: From Identification to Molecular Mechanism.Plants (Basel). 2024 Jul 19;13(14):1973. doi: 10.3390/plants13141973. Plants (Basel). 2024. PMID: 39065500 Free PMC article.
-
Phytoplasma DNA Enrichment from Sugarcane White Leaves for Shotgun Sequencing Improvement.Plants (Basel). 2024 Oct 28;13(21):3006. doi: 10.3390/plants13213006. Plants (Basel). 2024. PMID: 39519924 Free PMC article.
References
-
- Hanboonsong Y., Wangkeeree J., Kobori Y. Integrated management of the vectors of sugarcane white leaf disease in Thailand: An update. Int. Sugar J. 2017;119:220–223.
-
- Matsumoto T., Lee C., Teng W. Studies on sugarcane white leaf disease of Taiwan, with special reference to the transmission by a leafhopper, Epitettix hiroglyphicus Mats. Jpn. J. Phytopathol. 1969;35:251–259. doi: 10.3186/jjphytopath.35.251. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

