Autophagy markers LC3 and p62 in aging lumbar motor neurons
- PMID: 38885913
- PMCID: PMC11326290
- DOI: 10.1016/j.exger.2024.112483
Autophagy markers LC3 and p62 in aging lumbar motor neurons
Abstract
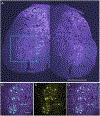
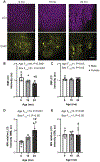
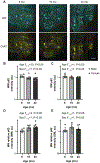
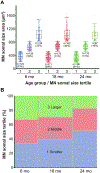
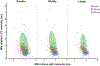
Autophagy is a ubiquitous process through which damaged cytoplasmic structures are recycled and degraded within cells. Aging can affect autophagy regulation in different steps leading to the accumulation of damaged organelles and proteins, which can contribute to cell dysfunction and death. Motor neuron (MN) loss and sarcopenia are prominent features of neuromuscular aging. Previous studies on phrenic MNs showed increased levels of the autophagy proteins LC3 and p62 in 24 month compared to 6 month old mice, consistent with the onset of diaphragm muscle sarcopenia. In the present study, we hypothesized that aging leads to increased expression of the autophagy markers LC3 and p62 in single lumbar MNs. Expression of LC3 and p62 in lumbar MNs (spinal levels L1-L6) was assessed using immunofluorescence and confocal imaging of male and female mice at 6, 18 and 24 months of age, reflecting 100 %, 90 % and 75 % survival, respectively. A mixed linear model with animal as a random effect was used to compare relative LC3 and p62 expression in choline acetyl transferase-positive MNs across age groups. Expression of LC3 and p62 decreased in the white matter of the lumbar spinal cord with aging, with ~29 % decrease in LC3 and ~ 7 % decrease in p62 expression at 24 months of age compared to 6 months of age. There was no change in LC3 or p62 expression in the gray matter with age. LC3 expression in MNs relative to white matter increased significantly with age, with 150 % increase at 24 months of age compared to 6 months of age. Similarly, p62 expression in MNs relative to white matter increased significantly with age, with ~14 % increase at 24 months of age compared to 6 months of age. No effect of sex or MN pool was observed in LC3 and p62 expression in MNs. Overall, these data suggest autophagy impairment during elongation (increased LC3) and degradation (increased p62) phases with aging in lumbar MNs.
Keywords: Aging; Autophagy; Motor neuron; Neuromuscular dysfunction; Spinal cord.
Copyright © 2024. Published by Elsevier Inc.
Conflict of interest statement
Declaration of competing interest This work was funded by NIH grant R01 AG057052 and the Mayo Clinic.
Figures
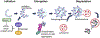
Similar articles
-
Age-related impairment of autophagy in cervical motor neurons.Exp Gerontol. 2021 Feb;144:111193. doi: 10.1016/j.exger.2020.111193. Epub 2020 Dec 5. Exp Gerontol. 2021. PMID: 33290859 Free PMC article.
-
Accumulation of p62 in degenerated spinal cord under chronic mechanical compression: functional analysis of p62 and autophagy in hypoxic neuronal cells.Autophagy. 2011 Dec;7(12):1462-71. doi: 10.4161/auto.7.12.17892. Autophagy. 2011. PMID: 22082874 Free PMC article.
-
Induction of autophagy and autophagic cell death in damaged neural tissue after acute spinal cord injury in mice.Spine (Phila Pa 1976). 2011 Oct 15;36(22):E1427-34. doi: 10.1097/BRS.0b013e3182028c3a. Spine (Phila Pa 1976). 2011. PMID: 21304420
-
Rapamycin treatment augments motor neuron degeneration in SOD1(G93A) mouse model of amyotrophic lateral sclerosis.Autophagy. 2011 Apr;7(4):412-25. doi: 10.4161/auto.7.4.14541. Epub 2011 Apr 1. Autophagy. 2011. PMID: 21193837
-
Natural Autophagy Activators to Fight Age-Related Diseases.Cells. 2024 Sep 26;13(19):1611. doi: 10.3390/cells13191611. Cells. 2024. PMID: 39404375 Free PMC article. Review.
Cited by
-
Molecular Mechanisms of Autophagy Decline during Aging.Cells. 2024 Aug 16;13(16):1364. doi: 10.3390/cells13161364. Cells. 2024. PMID: 39195254 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

