First-line bevacizumab plus chemotherapy in Chinese patients with stage III/IV epithelial ovarian cancer, fallopian tube cancer or primary peritoneal cancer: a phase III randomized controlled trial
- PMID: 38872480
- PMCID: PMC11390243
- DOI: 10.3802/jgo.2024.35.e99
First-line bevacizumab plus chemotherapy in Chinese patients with stage III/IV epithelial ovarian cancer, fallopian tube cancer or primary peritoneal cancer: a phase III randomized controlled trial
Abstract
Objective: First-line bevacizumab plus carboplatin and paclitaxel (CP) is approved for stage III/IV ovarian cancer treatment following initial surgical resection, based on global phase III GOG-0218 and ICON7 trials. This study evaluated the efficacy and safety of bevacizumab + CP as first-line ovarian cancer therapy in Chinese patients.
Methods: Patients with newly diagnosed, International Federation of Gynecology and Obstetrics (FIGO) stage III/IV epithelial ovarian, fallopian tube, or primary peritoneal cancer post-primary surgery were randomized 1:1 to receive 6 cycles of CP with bevacizumab/placebo, followed by bevacizumab/placebo maintenance until unacceptable toxicity or disease progression. Primary endpoint was investigator-assessed progression-free survival (PFS). Stratification factors were FIGO stage and debulking status (stage III optimally debulked vs stage III suboptimally debulked vs stage IV) and Eastern Cooperative Oncology Group performance status (0 vs 1 or 2).
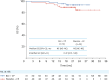
Results: Of randomized patients, 51 received bevacizumab + CP and 49 received placebo + CP. Median PFS was 22.6 months with bevacizumab + CP (95% confidence interval [CI]=18.6, not estimable) and 12.3 months (95% CI=9.5, 15.0) with placebo + CP (stratified hazard ratio=0.30; 95% CI=0.17, 0.53). Treatment-related grade 3/4 adverse events occurred in 46 of 49 (94%) patients receiving bevacizumab + CP, and 34 of 50 (68%) receiving placebo + CP.
Conclusion: Bevacizumab + CP showed clinically meaningful improvement in PFS vs placebo + CP, consistent with GOG-0218 results. Safety data were aligned with the known bevacizumab safety profile. These results support first-line bevacizumab + CP therapy in Chinese patients with ovarian cancer.
Trial registration: ClinicalTrials.gov Identifier: NCT03635489.
Keywords: Bevacizumab; Chemotherapy; Chinese; Ovarian Cancer.
© 2024. Asian Society of Gynecologic Oncology, Korean Society of Gynecologic Oncology, and Japan Society of Gynecologic Oncology.
Conflict of interest statement
W.X., L.J., A.R., Y.R., Z.Y., Z.H., H.A., W.L., Z.J., L.Z., D.W., Z.J., L.G., C.G., C.Y., and X.F. received institute research funding from F. Hoffmann-La Roche Ltd. N.S., W.H., and L.D. are employees of F. Hoffmann-La Roche Ltd. N.S. and W.H. own shares in F. Hoffmann-La Roche Ltd.
Figures

Similar articles
-
Trebananib or placebo plus carboplatin and paclitaxel as first-line treatment for advanced ovarian cancer (TRINOVA-3/ENGOT-ov2/GOG-3001): a randomised, double-blind, phase 3 trial.Lancet Oncol. 2019 Jun;20(6):862-876. doi: 10.1016/S1470-2045(19)30178-0. Epub 2019 May 7. Lancet Oncol. 2019. PMID: 31076365 Clinical Trial.
-
Weekly dose-dense chemotherapy in first-line epithelial ovarian, fallopian tube, or primary peritoneal carcinoma treatment (ICON8): primary progression free survival analysis results from a GCIG phase 3 randomised controlled trial.Lancet. 2019 Dec 7;394(10214):2084-2095. doi: 10.1016/S0140-6736(19)32259-7. Epub 2019 Nov 29. Lancet. 2019. PMID: 31791688 Free PMC article. Clinical Trial.
-
Atezolizumab, Bevacizumab, and Chemotherapy for Newly Diagnosed Stage III or IV Ovarian Cancer: Placebo-Controlled Randomized Phase III Trial (IMagyn050/GOG 3015/ENGOT-OV39).J Clin Oncol. 2021 Jun 10;39(17):1842-1855. doi: 10.1200/JCO.21.00306. Epub 2021 Apr 23. J Clin Oncol. 2021. PMID: 33891472 Free PMC article. Clinical Trial.
-
Expert consensus: Profiling and management of advanced or metastatic epithelial ovarian cancer.Rev Colomb Obstet Ginecol. 2024 Jun 14;75(1):4094. doi: 10.18597/rcog.4094. Rev Colomb Obstet Ginecol. 2024. PMID: 39013199 Free PMC article. English, Spanish.
-
Bevacizumab combination therapy: a review of its use in patients with epithelial ovarian, fallopian tube, or primary peritoneal cancer.BioDrugs. 2013 Aug;27(4):375-92. doi: 10.1007/s40259-013-0043-4. BioDrugs. 2013. PMID: 23728884 Review.
References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. - PubMed
-
- Ebell MH, Culp MB, Radke TJ. A systematic review of symptoms for the diagnosis of ovarian cancer. Am J Prev Med. 2016;50:384–394. - PubMed
-
- Karam A, Ledermann JA, Kim JW, Sehouli J, Lu K, Gourley C, et al. Fifth Ovarian Cancer Consensus Conference of the Gynecologic Cancer InterGroup: first-line interventions. Ann Oncol. 2017;28:711–717. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

