Effects of carvedilol on human prostate tissue contractility and stromal cell growth pointing to potential clinical implications
- PMID: 38858312
- PMCID: PMC11294394
- DOI: 10.1007/s43440-024-00605-5
Effects of carvedilol on human prostate tissue contractility and stromal cell growth pointing to potential clinical implications
Abstract
Background: Apart from antagonizing ß-adrenoceptors, carvedilol antagonizes vascular α1-adrenoceptors and activates G protein-independent signaling. Even though it is a commonly used antihypertensive and α1-adrenoceptors are essential for the treatment of voiding symptoms in benign prostatic hyperplasia, its actions in the human prostate are still unknown. Here, we examined carvedilol effects on contractions of human prostate tissues, and on stromal cell growth.
Methods: Contractions of prostate tissues from radical prostatectomy were induced by electric field stimulation (EFS) or α1-agonists. Growth-related functions were examined in cultured stromal cells.
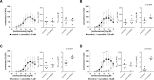
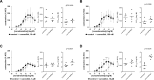
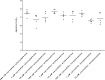
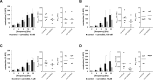
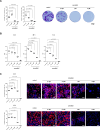
Results: Concentration-response curves for phenylephrine, methoxamine and noradrenaline were right shifted by carvedilol (0.1-10 µM), around half a magnitude with 100 nM, half to one magnitude with 1 µM, and two magnitudes with 10 µM. Right shifts were reflected by increased EC50 values for agonists, with unchanged Emax values. EFS-induced contractions were reduced by 21-54% with 0.01-1 µM carvedilol, and by 94% by 10 µM. Colony numbers of stromal cells were increased by 500 nM, but reduced by 1-10 µM carvedilol, while all concentrations reduced colony size. Decreases in viability were time-dependent with 0.1-0.3 µM, but complete with 10 µM. Proliferation was slightly increased by 0.1-0.5 µM, but reduced with 1-10 µM.
Conclusions: Carvedilol antagonizes α1-adrenoceptors in the human prostate, starting with concentrations in ranges of known plasma levels. In vitro, effect sizes resemble those of α1-blockers used for the treatment of voiding symptoms, which requires concentrations beyond plasma levels. Bidirectional and dynamic effects on the growth of stromal cells may be attributed to "biased agonism".
Keywords: Alpha1-blocker; Benign prostatic hyperplasia (BPH); Carvedilol; Lower urinary tract symptoms (LUTS); Prostate smooth muscle contraction; Voiding symptoms.
© 2024. The Author(s).
Conflict of interest statement
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Figures

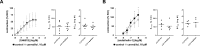
Similar articles
-
Inhibition of human prostate stromal cell growth and smooth muscle contraction by thalidomide: A novel remedy in LUTS?Prostate. 2021 May;81(7):377-389. doi: 10.1002/pros.24114. Epub 2021 Mar 9. Prostate. 2021. PMID: 33687083
-
Antagonism of α1-adrenoceptors by β3-adrenergic agonists: Structure-function relations of different agonists in prostate smooth muscle contraction.Biochem Pharmacol. 2022 Aug;202:115148. doi: 10.1016/j.bcp.2022.115148. Epub 2022 Jun 15. Biochem Pharmacol. 2022. PMID: 35716783
-
New strategies for inhibition of non-adrenergic prostate smooth muscle contraction by pharmacologic intervention.Prostate. 2019 May;79(7):746-756. doi: 10.1002/pros.23780. Epub 2019 Feb 27. Prostate. 2019. PMID: 30811062
-
Prostatic α1-adrenoceptors: new concepts of function, regulation, and intracellular signaling.Neurourol Urodyn. 2014 Sep;33(7):1074-85. doi: 10.1002/nau.22467. Epub 2013 Aug 22. Neurourol Urodyn. 2014. PMID: 24038148 Review.
-
The concept of uroselectivity.Eur Urol. 1998;33 Suppl 2:7-11. doi: 10.1159/000052228. Eur Urol. 1998. PMID: 9556190 Review.
References
-
- Gravas S, Gacci M, Gratzke C, Herrmann TRW, Karavitakis M, Kyriazis I, et al. Summary paper on the 2023 European Association of Urology Guidelines on the management of non-neurogenic male lower urinary tract symptoms. Eur Urol. 2023;84(2):207–22. 10.1016/j.eururo.2023.04.008. 10.1016/j.eururo.2023.04.008 - DOI - PubMed
-
- Ali M, Landeira M, Covernton PJO, Choudhury N, Jaggi A, Fatoye F, et al. The use of mono- and combination drug therapy in men and women with lower urinary tract symptoms (LUTS) in the UK: a retrospective observational study. BMC Urol. 2021;21(1):119. 10.1186/s12894-021-00881-w. 10.1186/s12894-021-00881-w - DOI - PMC - PubMed
-
- Cindolo L, Pirozzi L, Fanizza C, Romero M, Tubaro A, Autorino R, et al. Drug adherence and clinical outcomes for patients under pharmacological therapy for lower urinary tract symptoms related to benign prostatic hyperplasia: population-based cohort study. Eur Urol. 2015;68(3):418–25. 10.1016/j.eururo.2014.11.006. 10.1016/j.eururo.2014.11.006 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
